YKL-40, a secreted glycoprotein, promotes tumor angiogenesis
- PMID: 19767768
- PMCID: PMC2795793
- DOI: 10.1038/onc.2009.292
YKL-40, a secreted glycoprotein, promotes tumor angiogenesis
Abstract
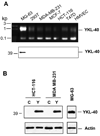
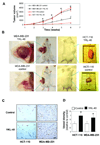
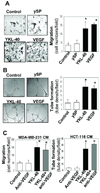
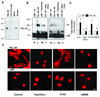
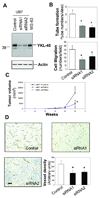
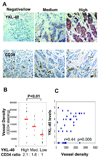
Tumor angiogenesis is of paramount importance in solid tumor development. Elevated serum levels of YKL-40, which is a secreted heparin-binding glycoprotein, have been associated with a worse prognosis from various advanced human cancers. Yet the role of YKL-40 activity in these cancers is still missing. In this study, we showed that ectopic expression of YKL-40 in MDA-MB-231 breast cancer cells and in HCT-116 colon cancer cells led to larger tumor formation with an extensive angiogenic phenotype than did control cancer cells in mice. Affinity-purified recombinant YKL-40 protein promoted vascular endothelial cell angiogenesis in vitro, the effects of which are similar to the activities observed using MDA-MB-231 and HCT-116 cell-conditioned medium after transfection with YKL-40. Furthermore, YKL-40 was found to induce coordination of membrane-bound receptor syndecan-1 and integrin alpha(v)beta(3) and to activate an intracellular signaling cascade, including focal adhesion kinase and mitogen-activated protein kinase extracellular signal-related kinase1/2 in endothelial cells. Moreover, blockade of YKL-40 using small-interfering RNA gene knockdown suppressed tumor angiogenesis in vitro and in vivo. Immunohistochemical analysis of human breast cancer showed a correlation between YKL-40 expression and blood vessel density. These findings provide novel insights into angiogenic activities and molecular mechanisms of YKL-40 in cancer development.
Conflict of interest statement
No potential conflicts of interest were disclosed.
Figures

References
-
- Albini A, Benelli R, Presta M, Rusnati M, Ziche M, Rubartelli A, Paglialunga G, Bussolino F, Noonan D. HIV-tat protein is a heparin-binding angiogenic growth factor. Oncogene. 1996;12:289–297. - PubMed
-
- Beauvais DM, Rapraeger AC. Syndecan-1-mediated cell spreading requires signaling by alphavbeta3 integrins in human breast carcinoma cells. Experimental Cell Research. 2003;286:219–232. - PubMed
-
- Bergmann OJ, Johansen JS, Klausen TW, Mylin AK, Kristensen JS, Kjeldsen E, Johnsen HE. High serum concentration of YKL-40 is associated with short survival in patients with acute myeloid leukemia. Clinical Cancer Research. 2005;11:8644–8652. - PubMed
-
- Bernfield M, Gotte M, Park PW, Reizes O, Fitzgerald ML, Lincecum J, Zako M. Functions of cell surface heparan sulfate proteoglycans. Annual Review of Biochemistry. 1999;68:729–777. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

