Medical and genetic differences in the adverse impact of sleep loss on performance: ethical considerations for the medical profession
- PMID: 19768182
- PMCID: PMC2744509
Medical and genetic differences in the adverse impact of sleep loss on performance: ethical considerations for the medical profession
Abstract
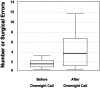
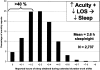
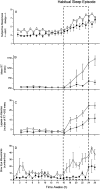
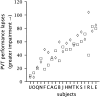
The Institute of Medicine recently concluded that-on average-medical residents make more serious medical errors and have more motor vehicle crashes when they are deprived of sleep. In the interest of public safety, society has required limitations on work hours in many other safety sensitive occupations, including transportation and nuclear power generation. Those who argue in favor of traditional extended duration resident work hours often suggest that there are inter- individual differences in response to acute sleep loss or chronic sleep deprivation, implying that physicians may be more resistant than the average person to the detrimental effects of sleep deprivation on performance, although there is no evidence that physicians are particularly resistant to such effects. Indeed, recent investigations have identified genetic polymorphisms that may convey a relative resistance to the effects of prolonged wakefulness on a subset of the healthy population, although there is no evidence that physicians are over-represented in this cohort. Conversely, there are also genetic polymorphisms, sleep disorders and other inter-individual differences that appear to convey an increased vulnerability to the performance-impairing effects of 24 hours of wakefulness. Given the magnitude of inter-individual differences in the effect of sleep loss on cognitive performance, and the sizeable proportion of the population affected by sleep disorders, hospitals face a number of ethical dilemmas. How should the work hours of physicians be limited to protect patient safety optimally? For example, some have argued that, in contrast to other professions, work schedules that repeatedly induce acute and chronic sleep loss are uniquely essential to the training of physicians. If evidence were to prove this premise to be correct, how should such training be ethically accomplished in the quartile of physicians and surgeons who are most vulnerable to the effects of sleep loss on performance without unacceptably compromising patient safety? Moreover, once it is possible to identify reliably those most vulnerable to the adverse effects of sleep loss on performance, will academic medical centers have an obligation to evaluate the proficiency of both residents and staff physicians under conditions of acute and chronic sleep deprivation? Should work-hour policy limits be modified to ensure that they are not hazardous for the patients of the most vulnerable quartile of physicians, or should the limits be personalized to enable the most resistant quartile to work longer hours? Given that the prevalence of sleep disorders has increased in our society overall, and increases markedly with age, how should fitness for extended duration work hours be monitored over a physician's career? In the spirit of the dictum to do no harm, advances in understanding the medical and genetic basis of inter-individual differences in the performance vulnerability to sleep loss should be incorporated into the development of work-hour policy limits for both physicians and surgeons.
Conflict of interest statement
Potential Conflicts of Interest: Dr. Czeisler is/was a consultant for: Actelion, Ltd.; Cephalon, Inc.; Delta Air Lines, Inc.; Eli Lilly and Co.; Garda Síochana Inspectorate; Global Ground Support; Johnson & Johnson; Koninklijke Philips Electronics, N.V.; Portland Trail Blazers; Respironics, Inc; Sanofi-Aventis Groupe; Sepracor, Inc.; Sleep Multimedia, Inc.; Somnus Therapeutics, Inc.; University of Wisconsin; Vanda Pharmaceuticals, Inc.; and Zeo, Inc.; and received royalties from McGraw Hill and Penguin Press. Dr. Czeisler owns an equity interest in Lifetrac, Inc.; Somnus Therapeutics, Inc.; Vanda Pharmaceuticals, Inc.; and Zeo, Inc. Dr. Czeisler has also received research support from Cephalon, Inc.; Tempur Pedic International, Inc; and Resmed, Inc. The Sleep and Health Education Program of the Harvard Medical School Division of Sleep Medicine has received support from Cephalon, Inc.; Takeda Pharmaceuticals North America, Inc.; Sanofi-Aventis Groupe; and Sepracor, Inc. Dr. Czeisler has received awards with monetary stipends from the American Clinical and Climatological Association; American Academy of Sleep Medicine; Association for Patient- Oriented Research; National Institute for Occupational Safety and Health and National Sleep Foundation; and the Sleep Research Society. Dr. Czeisler is the incumbent of an endowed professorship provided to Harvard University by Cephalon, Inc. and holds a number of process patents in the field of sleep/circadian rhythms (e.g., photic resetting of the human circadian pacemaker). Since 1985, Dr. Czeisler has also served as an expert witness on various legal cases related to sleep and/or circadian rhythms.
Figures





References
-
- Ulmer C, Wolman DM, Johns MME, editors. Resident Duty Hours: Enhancing Sleep, Supervision, and Safety. Washington, D.C.: The National Academies Press; 2008. Institute of Medicine of the National Academies; pp. 1–322. - PubMed
-
- Colten HR, Alteveogt BM, editors. Washington, D.C.: National Academies Press; 2006. Institute of Medicine. Sleep disorders and sleep deprivation: An unmet public health problem. . ISBN:0-309-66012-2. 1–500. - PubMed
-
- Assuring Quality Patient care and Quality Education. Association of American Medical Colleges; 2001. AAMC; pp. 1–10. - PubMed
-
- Friedman RC, Bigger JT, Kornfield DS. The intern and sleep loss. N Engl J Med. 1971;285:201–3. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- U18 HS015906/HS/AHRQ HHS/United States
- K08 HS013333/HS/AHRQ HHS/United States
- F33- HL-09588/HL/NHLBI NIH HHS/United States
- K08 HS13333/HS/AHRQ HHS/United States
- P01 AG09975/AG/NIA NIH HHS/United States
- T32 HL007901/HL/NHLBI NIH HHS/United States
- R01 AG006072/AG/NIA NIH HHS/United States
- R01 HL052992/HL/NHLBI NIH HHS/United States
- F32 HS14130/HS/AHRQ HHS/United States
- R01 MH-45130/MH/NIMH NIH HHS/United States
- R01 OH07567/OH/NIOSH CDC HHS/United States
- WT_/Wellcome Trust/United Kingdom
- T32 HL07901/HL/NHLBI NIH HHS/United States
- 060018/B/99/Z/WT_/Wellcome Trust/United Kingdom
- R01 HS012032/HS/AHRQ HHS/United States
- R01 HS12032/HS/AHRQ HHS/United States
- R01 HL52992/HL/NHLBI NIH HHS/United States
- M01 RR002635/RR/NCRR NIH HHS/United States
- M01 RR02635/RR/NCRR NIH HHS/United States
- R01 OH007567/OH/NIOSH CDC HHS/United States
- R01 MH045130/MH/NIMH NIH HHS/United States
- R01 AG06072/AG/NIA NIH HHS/United States
- F33 HL009588/HL/NHLBI NIH HHS/United States
- F32 HS014130/HS/AHRQ HHS/United States
- P01 AG009975/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
