Recombinant vectors as influenza vaccines
- PMID: 19768410
- PMCID: PMC7115000
- DOI: 10.1007/978-3-540-92165-3_13
Recombinant vectors as influenza vaccines
Abstract
The antiquated system used to manufacture the currently licensed inactivated influenza virus vaccines would not be adequate during an influenza virus pandemic. There is currently a search for vaccines that can be developed faster and provide superior, long-lasting immunity to influenza virus as well as other highly pathogenic viruses and bacteria. Recombinant vectors provide a safe and effective method to elicit a strong immune response to a foreign protein or epitope. This review explores the advantages and limitations of several different vectors that are currently being tested, and highlights some of the newer viruses being used as recombinant vectors.
Figures


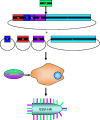

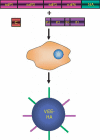
References
-
- Baric RS, Yount B, Lindesmith L, Harrington PR, Greene SR, Tseng FC, Davis N, Johnston RE, Klapper DG, Moe CL. Expression and self-assembly of Norwalk virus capsid protein from Venezuelan equine encephalitis virus replicons. J Virol. 2002;76(6):3023–3030. doi: 10.1128/JVI.76.6.3023-3030.2002. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

