Effects of the anesthetic agent propofol on neural populations
- PMID: 19768579
- PMCID: PMC2837528
- DOI: 10.1007/s11571-009-9092-2
Effects of the anesthetic agent propofol on neural populations
Abstract
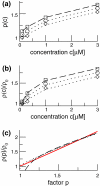
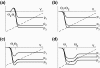
The neuronal mechanisms of general anesthesia are still poorly understood. Besides several characteristic features of anesthesia observed in experiments, a prominent effect is the bi-phasic change of power in the observed electroencephalogram (EEG), i.e. the initial increase and subsequent decrease of the EEG-power in several frequency bands while increasing the concentration of the anaesthetic agent. The present work aims to derive analytical conditions for this bi-phasic spectral behavior by the study of a neural population model. This model describes mathematically the effective membrane potential and involves excitatory and inhibitory synapses, excitatory and inhibitory cells, nonlocal spatial interactions and a finite axonal conduction speed. The work derives conditions for synaptic time constants based on experimental results and gives conditions on the resting state stability. Further the power spectrum of Local Field Potentials and EEG generated by the neural activity is derived analytically and allow for the detailed study of bi-spectral power changes. We find bi-phasic power changes both in monostable and bistable system regime, affirming the omnipresence of bi-spectral power changes in anesthesia. Further the work gives conditions for the strong increase of power in the δ-frequency band for large propofol concentrations as observed in experiments.
Figures




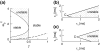





References
-
- Agmon-Sir H, Segev I. Signal delay and input synchronisation in passive dendritic structures. J Neurophysiol. 1993;70:2066–2085. - PubMed
-
- Amit DJ. Modeling brain function: the world of attactor neural networks. Cambridge: Cambridge University Press; 1989.
LinkOut - more resources
Full Text Sources

