Hydrocarbon hydroxylation by cytochrome P450 enzymes
- PMID: 19769330
- PMCID: PMC2820140
- DOI: 10.1021/cr9002193
Hydrocarbon hydroxylation by cytochrome P450 enzymes
Figures
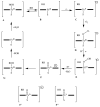

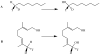
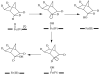


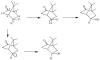
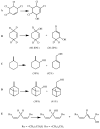
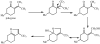

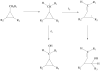



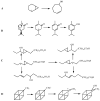
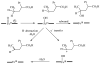
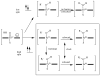
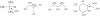
References
-
- Guengerich FP. In: Cytochrome P450: Structure, Mechanism, and Biochemistry. Ortiz de Montellano PR, editor. Kluwer Elsevier; New York: 2005. pp. 377–530.
-
- McLean KJ, Munro AW. Drug Metab Rev. 2008;40:427. - PubMed
-
- Ehlting J, Provart NJ, Werck-Reichhart D. Biochem Soc Trans. 2006;34:1192. - PubMed
-
- Schuler MA, Werck-Reichhart D. Annu Rev Plant Biol. 2003;54:629. - PubMed
-
- Nelson DR, Kamataki T, Waxman DJ, Guengerich FP, Estabrook RW, Feyereisen R, Gonzalez FJ, Coon MJ, Gunsalus IC, Gotoh O, Okuda K, Nebert DW. DNA Cell Biol. 1993;12:1. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

