Regulation of selenoproteins and methionine sulfoxide reductases A and B1 by age, calorie restriction, and dietary selenium in mice
- PMID: 19769460
- PMCID: PMC2864656
- DOI: 10.1089/ars.2009.2895
Regulation of selenoproteins and methionine sulfoxide reductases A and B1 by age, calorie restriction, and dietary selenium in mice
Abstract
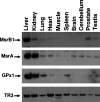
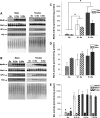
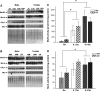
Methionine residues are susceptible to oxidation, but this damage may be reversed by methionine sulfoxide reductases MsrA and MsrB. Mammals contain one MsrA and three MsrBs, including a selenoprotein MsrB1. Here, we show that MsrB1 is the major methionine sulfoxide reductase in liver of mice and it is among the proteins that are most easily regulated by dietary selenium. MsrB1, but not MsrA activities, were reduced with age, and the selenium regulation of MsrB1 was preserved in the aging liver, suggesting that MsrB1 could account for the impaired methionine sulfoxide reduction in aging animals. We also examined regulation of Msr and selenoprotein expression by a combination of dietary selenium and calorie restriction and found that, under calorie restriction conditions, selenium regulation was preserved. In addition, mice overexpressing a mutant form of selenocysteine tRNA reduced MsrB1 activity to the level observed in selenium deficiency, whereas MsrA activity was elevated in these animals. Finally, we show that selenium regulation in inbred mouse strains is preserved in an outbred aging model. Taken together, these findings better define dietary regulation of methionine sulfoxide reduction and selenoprotein expression in mice with regard to age, calorie restriction, dietary Se, and a combination of these factors.
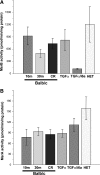
Figures


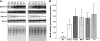
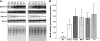
Similar articles
-
The methionine sulfoxide reduction system: selenium utilization and methionine sulfoxide reductase enzymes and their functions.Antioxid Redox Signal. 2013 Sep 20;19(9):958-69. doi: 10.1089/ars.2012.5081. Epub 2013 Jan 22. Antioxid Redox Signal. 2013. PMID: 23198996 Free PMC article. Review.
-
MsrB1 (methionine-R-sulfoxide reductase 1) knock-out mice: roles of MsrB1 in redox regulation and identification of a novel selenoprotein form.J Biol Chem. 2009 Feb 27;284(9):5986-93. doi: 10.1074/jbc.M805770200. Epub 2008 Nov 6. J Biol Chem. 2009. PMID: 18990697 Free PMC article.
-
Functions and evolution of selenoprotein methionine sulfoxide reductases.Biochim Biophys Acta. 2009 Nov;1790(11):1471-7. doi: 10.1016/j.bbagen.2009.04.014. Epub 2009 May 4. Biochim Biophys Acta. 2009. PMID: 19406207 Free PMC article. Review.
-
The selenoprotein methionine sulfoxide reductase B1 (MSRB1).Free Radic Biol Med. 2022 Oct;191:228-240. doi: 10.1016/j.freeradbiomed.2022.08.043. Epub 2022 Sep 7. Free Radic Biol Med. 2022. PMID: 36084791 Review.
-
Selenium and the methionine sulfoxide reductase system.Molecules. 2009 Jul 1;14(7):2337-44. doi: 10.3390/molecules14072337. Molecules. 2009. PMID: 19633607 Free PMC article. Review.
Cited by
-
The biological significance of methionine sulfoxide stereochemistry.Free Radic Biol Med. 2011 Jan 15;50(2):221-7. doi: 10.1016/j.freeradbiomed.2010.11.008. Epub 2010 Nov 11. Free Radic Biol Med. 2011. PMID: 21075204 Free PMC article. Review.
-
Selenoproteins: molecular pathways and physiological roles.Physiol Rev. 2014 Jul;94(3):739-77. doi: 10.1152/physrev.00039.2013. Physiol Rev. 2014. PMID: 24987004 Free PMC article. Review.
-
The methionine sulfoxide reduction system: selenium utilization and methionine sulfoxide reductase enzymes and their functions.Antioxid Redox Signal. 2013 Sep 20;19(9):958-69. doi: 10.1089/ars.2012.5081. Epub 2013 Jan 22. Antioxid Redox Signal. 2013. PMID: 23198996 Free PMC article. Review.
-
Methionine sulfoxide reductase B1 deficiency does not increase high-fat diet-induced insulin resistance in mice.Free Radic Res. 2017 Jan;51(1):24-37. doi: 10.1080/10715762.2016.1261133. Epub 2016 Dec 9. Free Radic Res. 2017. PMID: 27838938 Free PMC article.
-
Monitoring of Methionine Sulfoxide Content and Methionine Sulfoxide Reductase Activity.Methods Mol Biol. 2018;1661:285-299. doi: 10.1007/978-1-4939-7258-6_20. Methods Mol Biol. 2018. PMID: 28917052 Free PMC article.
References
-
- Baker RD. Baker SS. LaRosa K. Whitney C. Newburger PE. Selenium regulation of glutathione peroxidase in human hepatoma cell line Hep3B. Arch Biochem Biophys. 1993;304:53–57. - PubMed
-
- Berry MJ. Banu L. Chen YY. Mandel SJ. Kieffer JD. Harney JW. Larsen PR. Recognition of UGA as a selenocysteine codon in type I deiodinase requires sequences in the 3' untranslated region. Nature. 1991;353:273–276. - PubMed
-
- Brot N. Weissbach H. Biochemistry and physiological role of methionine sulfoxide residues in proteins. Arch Biochem Biophys. 1983;223:271–281. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

