Bench-to-bedside review: Diaphragm muscle function in disuse and acute high-dose corticosteroid treatment
- PMID: 19769782
- PMCID: PMC2784339
- DOI: 10.1186/cc7971
Bench-to-bedside review: Diaphragm muscle function in disuse and acute high-dose corticosteroid treatment
Abstract
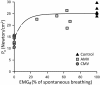
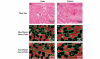
Critically ill patients may require mechanical ventilatory support and short-term high-dose corticosteroid to treat some specific underlying disease processes. Diaphragm muscle inactivity induced by controlled mechanical ventilation produces dramatic alterations in diaphragm muscle structure and significant losses in function. Although the exact mechanisms responsible for losses in diaphragm muscle function are still unknown, recent studies have highlighted the importance of proteolysis and oxidative stress. In experimental animals, short-term strategies that maintain partial diaphragm muscle neuromechanical activation mitigate diaphragmatic force loss. In animal models, studies on the influence of combined controlled mechanical ventilation and short-term high-dose methylprednisolone have given inconsistent results in regard to the effects on diaphragm muscle function. In the critically ill patient, further research is needed to establish the prevalence and mechanisms of ventilator-induced diaphragm muscle dysfunction, and the possible interaction between mechanical ventilation and the administration of high-dose corticosteroid. Until then, in caring for these patients, it is imperative to allow partial activation of the diaphragm, and to administer the lowest dose of corticosteroid for the shortest duration possible.
Figures



References
-
- Bracken MB, Shepard MJ, Holford TR, Leo-Summers L, Aldrich EF, Fazl M, Fehlings M, Herr DL, Hitchon PW, Marshall LF, Nockels RP, Pascale V, Perot PL Jr, Piepmeier J, Sonntag VK, Wagner F, Wilberger JE, Winn HR, Young W. Administration of methylprednisolone for 24 or 48 hours or tirilazad mesylate for 48 hours in the treatment of acute spinal cord injury. Results of the Third National Acute Spinal Cord Injury Randomized Controlled Trial. National Acute Spinal Cord Injury Study. JAMA. 1997;277:1597–1604. doi: 10.1001/jama.277.20.1597. - DOI - PubMed
-
- Kaplan PW, Rocha W, Sanders DB, D'Souza B, Spock A. Acute steroid-induced tetraplegia following status asthmaticus. Pediatrics. 1986;78:121–123. - PubMed

