Hormone and glucose signalling in POMC and AgRP neurons
- PMID: 19770186
- PMCID: PMC2793863
- DOI: 10.1113/jphysiol.2009.179192
Hormone and glucose signalling in POMC and AgRP neurons
Abstract
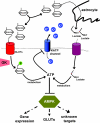
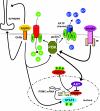
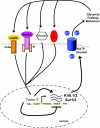
In the wake of the obesity pandemic, increased research efforts are under way to define how peripheral hormones and metabolites regulate energy homeostasis. The melanocortin system, comprising anorexigenic proopiomelanocortin (POMC) expressing neurons and orexigenic agouti-related protein (AgRP)/neuropeptide Y (NPY) coexpressing neurons in the arcuate nucleus of the hypothalamus are crucial for normal energy homeostasis both in rodents and humans. They are regulated by peripheral hormones such as leptin and insulin, as well as nutrients such as glucose, amino acids and fatty acids. Although much progress has been made, recent reports continue to underline how restricted our understanding of POMC and AgRP/NPY neuron regulation by these signals is. Importantly, ATP-dependent potassium (K(ATP)) channels are regulated both by ATP (from glucose metabolism) and by leptin and insulin, and directly control electrical excitability of both POMC and AgRP neurons. Thus, this review attempts to offer an integrative overview about how peripheral signals, particularly leptin, insulin and glucose, converge on a molecular level in POMC and AgRP neurons of the arcuate nucleus of the hypothalamus to control energy homeostasis.
Figures
References
-
- Acosta-Martinez M, Levine JE. Regulation of KATP channel subunit gene expression by hyperglycemia in the mediobasal hypothalamus of female rats. Am J Physiol Endocrinol Metab. 2007;292:E1801–1807. - PubMed
-
- Bady I, Marty N, Dallaporta M, Emery M, Gyger J, Tarussio D, Foretz M, Thorens B. Evidence from glut2-null mice that glucose is a critical physiological regulator of feeding. Diabetes. 2006;55:988–995. - PubMed
-
- Banks WA, Coon AB, Robinson SM, Moinuddin A, Shultz JM, Nakaoke R, Morley JE. Triglycerides induce leptin resistance at the blood-brain barrier. Diabetes. 2004;53:1253–1260. - PubMed
-
- Belgardt BF, Husch A, Rother E, Ernst MB, Wunderlich FT, Hampel B, Klockener T, Alessi D, Kloppenburg P, Bruning JC. PDK1 deficiency in POMC-expressing cells reveals FOXO1-dependent and -independent pathways in control of energy homeostasis and stress response. Cell Metab. 2008;7:291–301. - PubMed
-
- Bjorbaek C, Elmquist JK, Frantz JD, Shoelson SE, Flier JS. Identification of SOCS-3 as a potential mediator of central leptin resistance. Mol Cell. 1998;1:619–625. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

