The final step of hygromycin A biosynthesis, oxidation of C-5''-dihydrohygromycin A, is linked to a putative proton gradient-dependent efflux
- PMID: 19770276
- PMCID: PMC2786329
- DOI: 10.1128/AAC.01069-09
The final step of hygromycin A biosynthesis, oxidation of C-5''-dihydrohygromycin A, is linked to a putative proton gradient-dependent efflux
Abstract
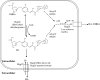
Hygromycin A (HA) is an aminocyclitol antibiotic produced and excreted by Streptomyces hygroscopicus. Deletion of hyg26 from the hygromycin A biosynthetic gene cluster has previously been shown to result in a mutant that produces 5''-dihydrohygromycin A (DHHA). We report herein on the purification and characterization of Hyg26 expressed in Escherichia coli. The enzyme catalyzes an NAD(H)-dependent reversible interconversion of HA and DHHA, supporting the role of the reduced HA as the penultimate biosynthetic pathway intermediate and not a shunt product. The equilibrium for the Hyg26-catalyzed reaction heavily favors the DHHA intermediate. The high-titer production of the HA product by S. hygroscopicus must be dependent upon a subsequent energetically favorable enzyme-catalyzed process, such as the selective and efficient export of HA. hyg19 encodes a putative proton gradient-dependent transporter, and a mutant lacking this gene was observed to produce less HA and to produce the DHHA intermediate. The DHHA produced by either the Deltahyg19 or the Deltahyg26 mutant had slightly reduced activity against E. coli and reduced protein synthesis-inhibitory activity in vitro. The data indicate that Hyg26 and Hyg19 have evolved to produce and export the final potent HA product in a coordinated fashion.
Figures






Similar articles
-
An O-phosphotransferase catalyzes phosphorylation of hygromycin A in the antibiotic-producing organism Streptomyces hygroscopicus.Antimicrob Agents Chemother. 2008 Oct;52(10):3580-8. doi: 10.1128/AAC.00157-08. Epub 2008 Jul 21. Antimicrob Agents Chemother. 2008. PMID: 18644964 Free PMC article.
-
Production of hygromycin A analogs in Streptomyces hygroscopicus NRRL 2388 through identification and manipulation of the biosynthetic gene cluster.Chem Biol. 2006 Jul;13(7):753-64. doi: 10.1016/j.chembiol.2006.05.013. Chem Biol. 2006. PMID: 16873023
-
Biosynthesis of the aminocyclitol subunit of hygromycin A in Streptomyces hygroscopicus NRRL 2388.Chem Biol. 2009 Nov 25;16(11):1180-9. doi: 10.1016/j.chembiol.2009.10.013. Chem Biol. 2009. PMID: 19942141
-
Biosynthetic origin of hygromycin A.Antimicrob Agents Chemother. 2003 Jul;47(7):2065-71. doi: 10.1128/AAC.47.7.2065-2071.2003. Antimicrob Agents Chemother. 2003. PMID: 12821448 Free PMC article.
-
Antibiotic export: transporters involved in the final step of natural product production.Microbiology (Reading). 2019 Aug;165(8):805-818. doi: 10.1099/mic.0.000794. Epub 2019 Apr 9. Microbiology (Reading). 2019. PMID: 30964430 Review.
Cited by
-
Disruption of Transporters Affiliated with Enantio-Pyochelin Biosynthesis Gene Cluster of Pseudomonas protegens Pf-5 Has Pleiotropic Effects.PLoS One. 2016 Jul 21;11(7):e0159884. doi: 10.1371/journal.pone.0159884. eCollection 2016. PLoS One. 2016. PMID: 27442435 Free PMC article.
-
Crystallographic characterization of the ribosomal binding site and molecular mechanism of action of Hygromycin A.Nucleic Acids Res. 2015 Nov 16;43(20):10015-25. doi: 10.1093/nar/gkv975. Epub 2015 Oct 12. Nucleic Acids Res. 2015. PMID: 26464437 Free PMC article.
-
Characterization of the biosynthetic gene cluster (ata) for the A201A aminonucleoside antibiotic from Saccharothrix mutabilis subsp. capreolus.J Antibiot (Tokyo). 2017 Apr;70(4):404-413. doi: 10.1038/ja.2016.123. Epub 2016 Oct 12. J Antibiot (Tokyo). 2017. PMID: 27731336
-
Distinct tRNA Accommodation Intermediates Observed on the Ribosome with the Antibiotics Hygromycin A and A201A.Mol Cell. 2015 Jun 4;58(5):832-44. doi: 10.1016/j.molcel.2015.04.014. Epub 2015 May 28. Mol Cell. 2015. PMID: 26028538 Free PMC article.
References
-
- Altschul, S. F., W. Gish, W. Miller, E. W. Myers, and D. J. Lipman. 1990. Basic local alignment search tool. J. Mol. Biol. 215:403-410. - PubMed
-
- Barrasa, M. I., J. A. Tercero, R. A. Lacalle, and A. Jimenez. 1995. The ard1 gene from Streptomyces capreolus encodes a polypeptide of the ABC-transporters superfamily which confers resistance to the aminonucleoside antibiotic A201A. Eur. J. Biochem. 228:562-569. - PubMed
-
- Cundliffe, E. 1989. How antibiotic-producing organisms avoid suicide. Annu. Rev. Microbiol. 43:207-233. - PubMed
-
- Dinos, G., D. N. Wilson, Y. Teraoka, W. Szaflarski, P. Fucini, D. Kalpaxis, and K. H. Nierhaus. 2004. Dissecting the ribosomal inhibition mechanisms of edeine and pactamycin: the universally conserved residues G693 and C795 regulate P-site RNA binding. Mol. Cell 13:113-124. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

