Vincristine, actinomycin, and cyclophosphamide compared with vincristine, actinomycin, and cyclophosphamide alternating with vincristine, topotecan, and cyclophosphamide for intermediate-risk rhabdomyosarcoma: children's oncology group study D9803
- PMID: 19770373
- PMCID: PMC2773476
- DOI: 10.1200/JCO.2009.22.3768
Vincristine, actinomycin, and cyclophosphamide compared with vincristine, actinomycin, and cyclophosphamide alternating with vincristine, topotecan, and cyclophosphamide for intermediate-risk rhabdomyosarcoma: children's oncology group study D9803
Abstract
Purpose: The purpose of this study was to compare the outcome of patients with intermediate-risk rhabdomyosarcoma (RMS) treated with standard VAC (vincristine, dactinomycin, and cyclophosphamide) chemotherapy to that of patients treated with VAC alternating with vincristine, topotecan, and cyclophosphamide (VAC/VTC).
Patients and methods: Patients were randomly assigned to 39 weeks of VAC versus VAC/VTC; local therapy began after week 12. Patients with parameningeal RMS with intracranial extension (PME) were treated with VAC and immediate x-ray therapy. The primary study end point was failure-free survival (FFS). The study was designed with 80% power (5% two-sided alpha level) to detect an increase in 5-year FFS from 64% to 75% with VAC/VTC.
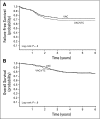
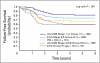
Results: A total of 617 eligible patients were entered onto the study: 264 were randomly assigned to VAC and 252 to VAC/VTC; 101 PME patients were nonrandomly treated with VAC. Treatment strata were embryonal RMS, stage 2/3, group III (33%); embryonal RMS, group IV, less than age 10 years (7%); alveolar RMS or undifferentiated sarcoma (UDS), stage 1 or group I (17%); alveolar RMS/UDS (27%); and PME (16%). At a median follow-up of 4.3 years, 4-year FFS was 73% with VAC and 68% with VAC/VTC (P = .3). There was no difference in effect of VAC versus VAC/VTC across risk groups. The frequency of second malignancies was similar between the two treatment groups.
Conclusion: For intermediate-risk RMS, VAC/VTC does not significantly improve FFS compared with VAC.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures


References
-
- Gurney JG, Young JL, Roffers SD, et al. Soft tissue sarcomas. In: Ries LAG, Smith MA, Gurney JG, et al., editors. Cancer Incidence and Survival Among Children and Adolescents: United States SEER Program 1975-1995. Bethesda, MD: 1999. pp. 111–123. NIH publication 99-4649.
-
- Raney RB, Anderson JR, Barr FG, et al. Rhabdomyosarcoma and undifferentiated sarcoma in the first two decades of life: A selective review of intergroup rhabdomyosarcoma study group experience and rationale for Intergroup Rhabdomyosarcoma Study V. J Pediatr Hematol Oncol. 2001;23:215–220. - PubMed
-
- Pappo AS, Shapiro DN, Crist WM, et al. Biology and therapy of pediatric rhabdomyosarcoma. J Clin Oncol. 1995;13:2123–2139. - PubMed
-
- Maurer HM, Beltangady M, Gehan EA, et al. The Intergroup Rhabdomyosarcoma Study-I. A final report. Cancer. 1988;61:209–220. - PubMed
-
- Maurer HM, Gehan EA, Beltangady M, et al. The Intergroup Rhabdomyosarcoma Study-II. Cancer. 1993;71:1904–1922. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

