Preoperative multimodality therapy improves disease-free survival in patients with carcinoma of the rectum: NSABP R-03
- PMID: 19770376
- PMCID: PMC2773471
- DOI: 10.1200/JCO.2009.22.0467
Preoperative multimodality therapy improves disease-free survival in patients with carcinoma of the rectum: NSABP R-03
Abstract
Purpose: Although chemoradiotherapy plus resection is considered standard treatment for operable rectal carcinoma, the optimal time to administer this therapy is not clear. The NSABP R-03 (National Surgical Adjuvant Breast and Bowel Project R-03) trial compared neoadjuvant versus adjuvant chemoradiotherapy in the treatment of locally advanced rectal carcinoma.
Patients and methods: Patients with clinical T3 or T4 or node-positive rectal cancer were randomly assigned to preoperative or postoperative chemoradiotherapy. Chemotherapy consisted of fluorouracil and leucovorin with 45 Gy in 25 fractions with a 5.40-Gy boost within the original margins of treatment. In the preoperative group, surgery was performed within 8 weeks after completion of radiotherapy. In the postoperative group, chemotherapy began after recovery from surgery but no later than 4 weeks after surgery. The primary end points were disease-free survival (DFS) and overall survival (OS).
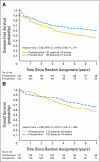
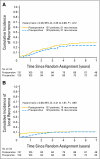
Results: From August 1993 to June 1999, 267 patients were randomly assigned to NSABP R-03. The intended sample size was 900 patients. Excluding 11 ineligible and two eligible patients without follow-up data, the analysis used data on 123 patients randomly assigned to preoperative and 131 to postoperative chemoradiotherapy. Surviving patients were observed for a median of 8.4 years. The 5-year DFS for preoperative patients was 64.7% v 53.4% for postoperative patients (P = .011). The 5-year OS for preoperative patients was 74.5% v 65.6% for postoperative patients (P = .065). A complete pathologic response was achieved in 15% of preoperative patients. No preoperative patient with a complete pathologic response has had a recurrence.
Conclusion: Preoperative chemoradiotherapy, compared with postoperative chemoradiotherapy, significantly improved DFS and showed a trend toward improved OS.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures
Comment in
-
Is preoperative chemoradiotherapy still the treatment of choice for rectal cancer?J Clin Oncol. 2009 Nov 1;27(31):5115-6. doi: 10.1200/JCO.2009.22.9112. Epub 2009 Sep 21. J Clin Oncol. 2009. PMID: 19770369 No abstract available.
-
Is the NSABP R-03 study in line with other chemoradiation studies?J Clin Oncol. 2010 Jun 20;28(18):e305-6; author reply e307. doi: 10.1200/JCO.2010.28.4356. Epub 2010 May 17. J Clin Oncol. 2010. PMID: 20479393 No abstract available.
References
-
- Colorectal Cancer Collaborative Group. Adjuvant radiotherapy for rectal cancer: A systematic overview of 8,507 patients from 22 randomized trials. Lancet. 2001;358:1291–1304. - PubMed
-
- Cammà C, Giunta M, Fiorica F, et al. Preoperative radiotherapy for resectable rectal cancer: A meta-analysis. JAMA. 2000;284:1008–1015. - PubMed
-
- Krook JE, Moertel CG, Gunderson LL, et al. Effective surgical adjuvant therapy for high-risk rectal carcinoma. N Engl J Med. 1991;324:709–715. - PubMed
-
- Wolmark N, Wieand HS, Hyams DM, et al. Randomized trial of postoperative adjuvant chemotherapy with or without radiotherapy for carcinoma of the rectum: National Surgical Adjuvant Breast and Bowel Project Protocol R-02. J Natl Cancer Inst. 2000;92:388–396. - PubMed
-
- Kapiteijn E, Marijnen CA, Nagtegaal ID, et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. N Engl J Med. 2001;345:638–646. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

