B cells produce pathogenic antibodies and impair recovery after spinal cord injury in mice
- PMID: 19770513
- PMCID: PMC2752085
- DOI: 10.1172/JCI39780
B cells produce pathogenic antibodies and impair recovery after spinal cord injury in mice
Abstract
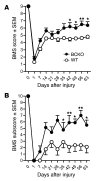
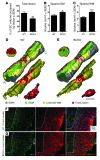
Traumatic injury to the mammalian spinal cord activates B cells, which culminates in the synthesis of autoantibodies. The functional significance of this immune response is unclear. Here, we show that locomotor recovery was improved and lesion pathology was reduced after spinal cord injury (SCI) in mice lacking B cells. After SCI, antibody-secreting B cells and Igs were present in the cerebrospinal fluid and/or injured spinal cord of WT mice but not mice lacking B cells. In mice with normal B cell function, large deposits of antibody and complement component 1q (C1q) accumulated at sites of axon pathology and demyelination. Antibodies produced after SCI caused pathology, in part by activating intraspinal complement and cells bearing Fc receptors. These data indicate that B cells, through the production of antibodies, affect pathology in SCI. One or more components of this pathologic immune response could be considered as novel therapeutic targets for minimizing tissue injury and/or promoting repair after SCI.
Figures




Comment in
-
Pathogenic antibodies are active participants in spinal cord injury.J Clin Invest. 2009 Oct;119(10):2881-4. doi: 10.1172/JCI40839. Epub 2009 Sep 21. J Clin Invest. 2009. PMID: 19770517 Free PMC article.
References
-
- Blight A.R. Delayed demyelination and macrophage invasion: a candidate for secondary cell damage in spinal cord injury. Cent. Nerv. Syst. Trauma. 1985;2:299–315. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

