CD4+CD25+Foxp3+ Tregs resolve experimental lung injury in mice and are present in humans with acute lung injury
- PMID: 19770521
- PMCID: PMC2752062
- DOI: 10.1172/JCI36498
CD4+CD25+Foxp3+ Tregs resolve experimental lung injury in mice and are present in humans with acute lung injury
Abstract
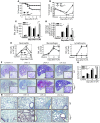
Acute lung injury (ALI) is characterized by rapid alveolar injury, inflammation, cytokine induction, and neutrophil accumulation. Although early events in the pathogenesis of ALI have been defined, the mechanisms underlying resolution are unknown. As a model of ALI, we administered intratracheal (i.t.) LPS to mice and observed peak lung injury 4 days after the challenge, with resolution by day 10. Numbers of alveolar lymphocytes increased as injury resolved. To examine the role of lymphocytes in this response, lymphocyte-deficient Rag-1-/- and C57BL/6 WT mice were exposed to i.t. LPS. The extent of injury was similar between the groups of mice through day 4, but recovery was markedly impaired in the Rag-1-/- mice. Adoptive transfer studies revealed that infusion of CD4+CD25+Foxp3+ Tregs as late as 24 hours after i.t. LPS normalized resolution in Rag-1-/- mice. Similarly, Treg depletion in WT mice delayed recovery. Treg transfer into i.t. LPS-exposed Rag-1-/- mice also corrected the elevated levels of alveolar proinflammatory cytokines and increased the diminished levels of alveolar TGF-beta and neutrophil apoptosis. Mechanistically, Treg-mediated resolution of lung injury was abrogated by TGF-beta inhibition. Moreover, BAL of patients with ALI revealed dynamic changes in CD3+CD4+CD25hiCD127loFoxp3+ cells. These results indicate that Tregs modify innate immune responses during resolution of lung injury and suggest potential targets for treating ALI, for which there are no specific therapies currently available.
Figures


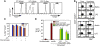


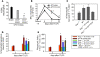


Comment in
-
Resolving lung injury: a new role for Tregs in controlling the innate immune response.J Clin Invest. 2009 Oct;119(10):2891-4. doi: 10.1172/JCI40880. Epub 2009 Sep 21. J Clin Invest. 2009. PMID: 19770510 Free PMC article.
References
-
- Savill J. Apoptosis in resolution of inflammation. J. Leukoc. Biol. 1997;61:375–380. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

