Antihypertensive treatment of acute cerebral hemorrhage
- PMID: 19770736
- PMCID: PMC5568798
- DOI: 10.1097/CCM.0b013e3181b9e1a5
Antihypertensive treatment of acute cerebral hemorrhage
Abstract
Objective: To determine the feasibility and acute (i.e., within 72 hrs) safety of three levels of systolic blood pressure reduction in subjects with supratentorial intracerebral hemorrhage treated within 6 hrs after symptom onset.
Design: A traditional phase I, dose-escalation, multicenter prospective study.
Settings: Emergency departments and intensive care units.
Patients: Patients with intracerebral hemorrhage with elevated systolic blood pressure > or = 170 mm Hg who present to the emergency department within 6 hrs of symptom onset.
Intervention: Intravenous nicardipine to reduce systolic blood pressure to a target of: (1) 170 to 200 mm Hg in the first cohort of patients; (2) 140 to 170 mm Hg in the second cohort; and (3) 110 to 140 mm Hg in the third cohort.
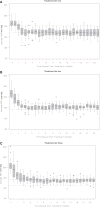
Measurements and main results: Primary outcomes of interest were: (1) treatment feasibility (achieving and maintaining the systolic blood pressure goals for 18-24 hrs); (2) neurologic deterioration within 24 hrs; and (3) serious adverse events within 72 hrs. Safety stopping rules based on neurologic deterioration and serious adverse events were prespecified and approved by an NIH-appointed Data and Safety Monitoring Board, which provided oversight on subject safety. Each subject was followed-up for 3 months to preliminarily assess mortality and the clinical outcomes. A total of 18, 20, and 22 patients were enrolled in the respective three tiers of systolic blood pressure treatment goals. Overall, 9 of 60 patients had treatment failures (all in the last tier). A total of seven subjects with neurologic deterioration were observed: one (6%), two (10%), and four (18%) in tier one, two, and three, respectively. Serious adverse events were observed in one subject (5%) in tier two and in three subjects (14%) in tier three. However, the safety stopping rule was not activated in any of the tiers. Three (17%), two (10%), and five (23%) subjects in tiers one, two, and three, respectively, died within 3 months.
Conclusions: The observed proportions of neurologic deterioration and serious adverse events were below the prespecified safety thresholds, and the 3-month mortality rate was lower than expected in all systolic blood pressure tiers. The results form the basis of a larger randomized trial addressing the efficacy of systolic blood pressure reduction in patients with intracerebral hemorrhage.
Conflict of interest statement
The authors have not disclosed any potential conflicts of interest.
Figures
Comment in
-
Blood pressure after intracerebral hemorrhage: lower may not be safer.Crit Care Med. 2010 Feb;38(2):731-2. doi: 10.1097/CCM.0b013e3181bfe9c3. Crit Care Med. 2010. PMID: 20083952 No abstract available.
References
-
- Qureshi AI. Acute hypertensive response in patients with stroke: pathophysiology and management. Circulation. 2008;118:176–187. - PubMed
-
- Qureshi AI, Suri MF, Nasar A, et al. Changes in cost and outcome among US patients with stroke hospitalized in 1990 to 1991 and those hospitalized in 2000 to 2001. Stroke. 2007;38:2180–2184. - PubMed
-
- Wallace JD, Levy LL. Blood pressure after stroke. JAMA. 1981;246:2177–2180. - PubMed
-
- Broderick JP, Brott TG, Tomsick T, et al. Ultra-early evaluation of intracerebral hemorrhage. J Neurosurg. 1990;72:195–199. - PubMed
-
- Chen ST, Chen SD, Hsu CY, et al. Progression of hypertensive intracerebral hemorrhage. Neurology. 1989;39:1509–1514. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical