Estrogen/estrogen receptor alpha signaling in mouse posterofrontal cranial suture fusion
- PMID: 19771170
- PMCID: PMC2743190
- DOI: 10.1371/journal.pone.0007120
Estrogen/estrogen receptor alpha signaling in mouse posterofrontal cranial suture fusion
Abstract
Background: While premature suture fusion, or craniosynostosis, is a relatively common condition, the cause is often unknown. Estrogens are associated with growth plate fusion of endochondral bones. In the following study, we explore the previously unknown significance of estrogen/estrogen receptor signaling in cranial suture biology.
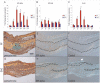
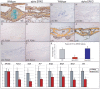
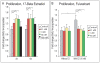
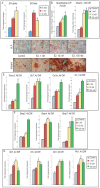
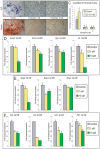
Methodology/principal findings: Firstly, estrogen receptor (ER) expression was examined in physiologically fusing (posterofrontal) and patent (sagittal) mouse cranial sutures by quantitative RT-PCR. Next, the cranial suture phenotype of ER alpha and ER beta knockout (alphaERKO, betaERKO) mice was studied. Subsequently, mouse suture-derived mesenchymal cells (SMCs) were isolated; the effects of 17-beta estradiol or the estrogen antagonist Fulvestrant on gene expression, osteogenic and chondrogenic differentiation were examined in vitro. Finally, in vivo experiments were performed in which Fulvestrant was administered subcutaneously to the mouse calvaria. Results showed that increased ERalpha but not ERbeta transcript abundance temporally coincided with posterofrontal suture fusion. The alphaERKO but not betaERKO mouse exhibited delayed posterofrontal suture fusion. In vitro, addition of 17-beta estradiol enhanced both osteogenic and chondrogenic differentiation in suture-derived mesenchymal cells, effects reversible by Fulvestrant. Finally, in vivo application of Fulvestrant significantly diminished calvarial osteogenesis, inhibiting suture fusion.
Conclusions/significance: Estrogen signaling through ERalpha but not ERbeta is associated with and necessary for normal mouse posterofrontal suture fusion. In vitro studies suggest that estrogens may play a role in osteoblast and/or chondrocyte differentiation within the cranial suture complex.
Conflict of interest statement
Figures
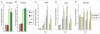

References
-
- Farkas LG, Tompson B, Phillips JH, Katic MJ, Cornfoot ML. Comparison of anthropometric and cephalometric measurements of the adult face. J Craniofac Surg. 1999;10:18–25; discussion 26. - PubMed
-
- Panchal J, Uttchin V. Management of craniosynostosis. Plast Reconstr Surg. 2003;111:2032–2048; quiz 2049. - PubMed
-
- Lenton KA, Nacamuli RP, Wan DC, Helms JA, Longaker MT. Cranial suture biology. Curr Top Dev Biol. 2005;66:287–328. - PubMed
-
- Sahar DE, Longaker MT, Quarto N. Sox9 neural crest determinant gene controls patterning and closure of the posterior frontal cranial suture. Dev Biol. 2005;280:344–361. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

