The fluorescent protein palette: tools for cellular imaging
- PMID: 19771335
- PMCID: PMC2910338
- DOI: 10.1039/b901966a
The fluorescent protein palette: tools for cellular imaging
Erratum in
- Chem Soc Rev. 2011 Dec;40(12):5923
Abstract
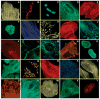
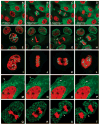
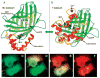
This critical review provides an overview of the continually expanding family of fluorescent proteins (FPs) that have become essential tools for studies of cell biology and physiology. Here, we describe the characteristics of the genetically encoded fluorescent markers that now span the visible spectrum from deep blue to deep red. We identify some of the novel FPs that have unusual characteristics that make them useful reporters of the dynamic behaviors of proteins inside cells, and describe how many different optical methods can be combined with the FPs to provide quantitative measurements in living systems (227 references).
Figures










Similar articles
-
Fluorescent protein tools for studying protein dynamics in living cells: a review.J Biomed Opt. 2008 May-Jun;13(3):031202. doi: 10.1117/1.2939093. J Biomed Opt. 2008. PMID: 18601526 Review.
-
Fluorescent proteins for FRET microscopy: monitoring protein interactions in living cells.Bioessays. 2012 May;34(5):341-50. doi: 10.1002/bies.201100098. Epub 2012 Mar 7. Bioessays. 2012. PMID: 22396229 Free PMC article. Review.
-
The structure and function of fluorescent proteins.Chem Soc Rev. 2009 Oct;38(10):2852-64. doi: 10.1039/b913033k. Epub 2009 Aug 21. Chem Soc Rev. 2009. PMID: 19771332 Review.
-
Live Cell Imaging and Confocal Microscopy.Methods Mol Biol. 2018;1789:117-130. doi: 10.1007/978-1-4939-7856-4_9. Methods Mol Biol. 2018. PMID: 29916075
-
A new configuration of the Zeiss LSM 510 for simultaneous optical separation of green and red fluorescent protein pairs.Cytometry A. 2006 Aug 1;69(8):920-9. doi: 10.1002/cyto.a.20323. Cytometry A. 2006. PMID: 16969813
Cited by
-
A transgenic pig model expressing a CMV-ZsGreen1 reporter across an extensive array of tissues.J Biomed Res. 2020 Dec 25;35(2):163-173. doi: 10.7555/JBR.34.20200111. J Biomed Res. 2020. PMID: 33797416 Free PMC article.
-
What is the Optimal Size of the Quantum Region in Embedding Calculations of Two-Photon Absorption Spectra of Fluorescent Proteins?J Chem Theory Comput. 2020 Oct 13;16(10):6439-6455. doi: 10.1021/acs.jctc.0c00602. Epub 2020 Sep 21. J Chem Theory Comput. 2020. PMID: 32862643 Free PMC article.
-
Förster resonance energy transfer microscopy and spectroscopy for localizing protein-protein interactions in living cells.Cytometry A. 2013 Sep;83(9):780-93. doi: 10.1002/cyto.a.22321. Epub 2013 Jun 27. Cytometry A. 2013. PMID: 23813736 Free PMC article. Review.
-
Acute manipulation of phosphoinositide levels in cells.Methods Cell Biol. 2012;108:187-207. doi: 10.1016/B978-0-12-386487-1.00010-9. Methods Cell Biol. 2012. PMID: 22325604 Free PMC article.
-
Heterogeneous Presynaptic Distribution of Munc13 Isoforms at Retinal Synapses and Identification of an Unconventional Bipolar Cell Type with Dual Expression of Munc13 Isoforms: A Study Using Munc13-EXFP Knock-in Mice.Int J Mol Sci. 2020 Oct 22;21(21):7848. doi: 10.3390/ijms21217848. Int J Mol Sci. 2020. PMID: 33105896 Free PMC article.
References
-
- Pliny, Bostock J, Riley HT. The natural history of Pliny, Book XXXII. Remedies derived from aquatic animals. Chapter 52—Other aquatic productions. Adarca or Calamochnos: three remedies. Reeds: eight remedies. The ink of the sæpia. Gaius Plinius Secundus (Pliny the Elder). AD77. H. G. Bohn; London: 1855.
-
- Matz MV, Lukyanov KA, Lukyanov SA. Bioessays. 2002;24:953–959. - PubMed
-
- Chalfie M, Kain S. Green fluorescent protein: properties, applications, and protocols. 2nd. Wiley-Interscience; Hoboken, NY: 2006.
-
- Shimomura O, Johnson FH, Saiga Y. J Cell Comp Physiol. 1962;59:223–239. - PubMed
-
- Morise H, Shimomura O, Johnson FH, Winant J. Biochemistry. 1974;13:2656–2662. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

