Asymmetric carbon-carbon bond formation gamma to a carbonyl group: phosphine-catalyzed addition of nitromethane to allenes
- PMID: 19772285
- PMCID: PMC2758928
- DOI: 10.1021/ja9061823
Asymmetric carbon-carbon bond formation gamma to a carbonyl group: phosphine-catalyzed addition of nitromethane to allenes
Abstract
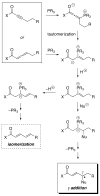
A chiral phosphine catalyzes the addition of a carbon nucleophile to the gamma position of an electron-poor allene (amide-, ester-, or phosphonate-substituted), in preference to isomerization to a 1,3-diene, in good ee and yield. This strategy provides an attractive method for the catalytic asymmetric gamma functionalization of carbonyl (and related) compounds.
Figures
References
-
-
For example, see reviews of: aldol reactions: Carreira EM, Fettes A, Marti C. Org Reactions. 2006;67:1–216.conjugate additions of Grignard reagents: Harutyunyan S, den Hartog T, Geurts K, Minnaard AJ, Feringa BL. Chem Rev. 2008;108:2824–2852.
-
-
-
For example, see a review of catalytic asymmetric vinylogous aldol reactions: Denmark SE, Heemstra JR, Jr, Beutner GL. Angew Chem, Int Ed. 2005;44:4682–4698.
-
-
-
Trost BM, Kazmaier U. J Am Chem Soc. 1992;114:7933–7935.For a review, see: Kwong CKW, Fu MY, Lam CSL, Toy PH. Synthesis. 2008:2307–2317.
-
-
- Trost BM, Li CJ. J Am Chem Soc. 1994;116:10819–10820.
- Trost BM, Li CJ. J Am Chem Soc. 1994;116:3167–3168.
- Trost BM, Dake GR. J Org Chem. 1997;62:5670–5671.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources