Microbial iron acquisition: marine and terrestrial siderophores
- PMID: 19772347
- PMCID: PMC2761978
- DOI: 10.1021/cr9002787
Microbial iron acquisition: marine and terrestrial siderophores
Figures


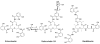
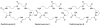


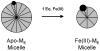
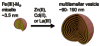
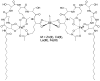
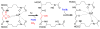
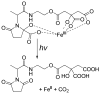

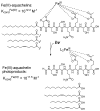
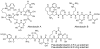

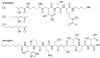

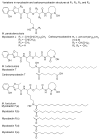
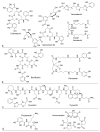

References
-
- Crosa JH, Mey AR, Payne SM, editors. Iron Transport In Bacteria. ASM Press; Washington DC: 2004.
-
- Templeton DM, editor. Molecular and Cellular Iron Transport. Marcel Dekker, Inc.; New York: 2002.
-
- Martin JH, Gorden RM. Deep Sea Research. 1988;35:117.
-
- Aguilar-Islas AM, Hurst MP, Buck KN, Sohst B, Smith GJ, Lohan MC, Bruland KW. Progress in Oceanography. 2007;73:99.
-
- Rue EL, Bruland KW. Mar Chem. 1995;50:117.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

