Branch-specific sialylation of IgG-Fc glycans by ST6Gal-I
- PMID: 19772356
- PMCID: PMC2761508
- DOI: 10.1021/bi901430h
Branch-specific sialylation of IgG-Fc glycans by ST6Gal-I
Abstract
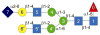
Sialylated forms of the Fc fragment of immunoglobulin G, produced by the human alpha2-6 sialyltransferase ST6Gal-I, were identified as potent anti-inflammatory mediators in a mouse model of rheumatoid arthritis and are potentially the active components in intravenous IgG anti-inflammatory therapies. The activities and specificities of hST6Gal-I are, however, poorly characterized. Here MS and NMR methodology demonstrates glycan modification occurs in a branch-specific manner with the alpha1-3Man branch of the complex, biantennary Fc glycan preferentially sialylated. Interestingly, this substrate preference is preserved when using a released glycan, suggesting that the apparent occlusion of glycan termini in Fc crystal structures does not dominate specificity.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

