Deoxyribonuclease I is essential for DNA fragmentation induced by gamma radiation in mice
- PMID: 19772469
- PMCID: PMC5075390
- DOI: 10.1667/RR1647.1
Deoxyribonuclease I is essential for DNA fragmentation induced by gamma radiation in mice
Abstract
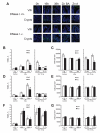
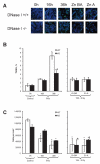
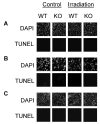
Gamma radiation is known to induce cell death in several organs. This damage is associated with endonuclease-mediated DNA fragmentation; however, the enzyme that produces the latter and is likely to cause cell death is unknown. To determine whether the most abundant cytotoxic endonuclease DNase I mediates gamma-radiation-induced tissue injury, we used DNase I knockout mice and zinc chelate of 3,5-diisopropylsalicylic acid (Zn-DIPS), which, as we show, has DNase I inhibiting activity in vitro. The study demonstrated for the first time that inactivation or inhibition of DNase I ameliorates radiation injury to the white pulp of spleen, intestine villi and bone marrow as measured using a quantitative TUNEL assay. The spleen and intestine of DNase I knockout mice were additionally protected from radiation by Zn-DIPS, perhaps due to the broad radioprotective effect of the zinc ions. Surprisingly, the main DNase I-producing tissues such as the salivary glands, pancreas and kidney showed no effect of DNase I inactivation. Another unexpected observation was that even without irradiation, DNA fragmentation and cell death were significantly lower in the intestine of DNase I knockout mice than in wild-type mice. This points to the physiological role of DNase I in normal cell death in the intestinal epithelium. In conclusion, our results suggested that DNase I-mediated mechanism of DNA damage and subsequent tissue injury are essential in gamma-radiation-induced cell death in radiosensitive organs.
Figures



References
-
- Skalka M, Matyasova J. The effect of radiation on deoxyribonucleoproteins in animal tissue. 3. The character of the polydeoxyribonucleotides released from irradiated tissues. Fol. Biol. (Praha) 1967;13:457–464. - PubMed
-
- Swingle KF, Cole LJ. Radiation-induced free polydeoxyribonucleotides in lymphoid tissues: A product of the action of neutral deoxyribonuclease (DNase 1) Radiat. Res. 1967;30:81–95. - PubMed
-
- Tabachnick J, LaBadie JH. Increased activity of skin surface DNase I after beta-irradiation injury or clipping of guinea pig hair. J. Invest. Dermatol. 1970;55:89–93. - PubMed
-
- Nakagami Y, Ito M, Hara T, Inoue T, Matsubara S. Nuclear translocation of DNase II and acid phosphatase during radiation-induced apoptosis in HL60 cells. Acta Oncol. 2003;42:227–236. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

