NMDA receptor subunit expression and PAR2 receptor activation in colospinal afferent neurons (CANs) during inflammation induced visceral hypersensitivity
- PMID: 19772634
- PMCID: PMC2758842
- DOI: 10.1186/1744-8069-5-54
NMDA receptor subunit expression and PAR2 receptor activation in colospinal afferent neurons (CANs) during inflammation induced visceral hypersensitivity
Abstract
Background: Visceral hypersensitivity is a clinical observation made when diagnosing patients with functional bowel disorders. The cause of visceral hypersensitivity is unknown but is thought to be attributed to inflammation. Previously we demonstrated that a unique set of enteric neurons, colospinal afferent neurons (CANs), co-localize with the NR1 and NR2D subunits of the NMDA receptor as well as with the PAR2 receptor. The aim of this study was to determine if NMDA and PAR2 receptors expressed on CANs contribute to visceral hypersensitivity following inflammation. Recently, work has suggested that dorsal root ganglion (DRG) neurons expressing the transient receptor potential vanilloid-1 (TRPV1) receptor mediate inflammation induced visceral hypersensitivity. Therefore, in order to study CAN involvement in visceral hypersensitivity, DRG neurons expressing the TRPV1 receptor were lesioned with resiniferatoxin (RTX) prior to inflammation and behavioural testing.
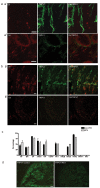
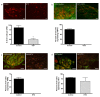
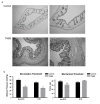
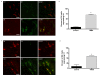
Results: CANs do not express the TRPV1 receptor; therefore, they survive following RTX injection. RTX treatment resulted in a significant decrease in TRPV1 expressing neurons in the colon and immunohistochemical analysis revealed no change in peptide or receptor expression in CANs following RTX lesioning as compared to control data. Behavioral studies determined that both inflamed non-RTX and RTX animals showed a decrease in balloon pressure threshold as compared to controls. Immunohistochemical analysis demonstrated that the NR1 cassettes, N1 and C1, of the NMDA receptor on CANs were up-regulated following inflammation. Furthermore, inflammation resulted in the activation of the PAR2 receptors expressed on CANs.
Conclusion: Our data show that inflammation causes an up-regulation of the NMDA receptor and the activation of the PAR2 receptor expressed on CANs. These changes are associated with a decrease in balloon pressure in response to colorectal distension in non-RTX and RTX lesioned animals. Therefore, these data suggest that CANs contribute to visceral hypersensitivity during inflammation.
Figures

Similar articles
-
Lesioning of TRPV1 expressing primary afferent neurons prevents PAR-2 induced motility, but not mechanical hypersensitivity in the rat colon.Neurogastroenterol Motil. 2012 Mar;24(3):e125-35. doi: 10.1111/j.1365-2982.2011.01848.x. Epub 2011 Dec 13. Neurogastroenterol Motil. 2012. PMID: 22168801 Free PMC article.
-
α2δ-1 Upregulation in Primary Sensory Neurons Promotes NMDA Receptor-Mediated Glutamatergic Input in Resiniferatoxin-Induced Neuropathy.J Neurosci. 2021 Jul 7;41(27):5963-5978. doi: 10.1523/JNEUROSCI.0303-21.2021. Epub 2021 Jun 17. J Neurosci. 2021. PMID: 34252037 Free PMC article.
-
Genesis of anxiety, depression, and ongoing abdominal discomfort in ulcerative colitis-like colon inflammation.Am J Physiol Regul Integr Comp Physiol. 2015 Jan 1;308(1):R18-27. doi: 10.1152/ajpregu.00298.2014. Epub 2014 Nov 19. Am J Physiol Regul Integr Comp Physiol. 2015. PMID: 25411361 Free PMC article.
-
Development, plasticity and modulation of visceral afferents.Brain Res Rev. 2009 Apr;60(1):171-86. doi: 10.1016/j.brainresrev.2008.12.004. Epub 2008 Dec 25. Brain Res Rev. 2009. PMID: 19150371 Free PMC article. Review.
-
[Recent Findings on the Mechanism of Cough Hypersensitivity as a Cause of Chronic Cough].Yakugaku Zasshi. 2021;141(12):1333-1342. doi: 10.1248/yakushi.21-00155. Yakugaku Zasshi. 2021. PMID: 34853206 Review. Japanese.
Cited by
-
Low-Affinity NMDA Receptor Antagonist Hemantane in a Topical Formulation Attenuates Arthritis Induced by Freund's Complete Adjuvant in Rats.Adv Pharm Bull. 2024 Mar;14(1):241-252. doi: 10.34172/apb.2024.002. Epub 2023 Jul 19. Adv Pharm Bull. 2024. PMID: 38585463 Free PMC article.
-
Electroacupuncture intervention of visceral hypersensitivity is involved in PAR-2-activation and CGRP-release in the spinal cord.Sci Rep. 2020 Jul 7;10(1):11188. doi: 10.1038/s41598-020-67702-2. Sci Rep. 2020. PMID: 32636402 Free PMC article.
-
Peripheral and Spinal Mechanisms Involved in Electro-Acupuncture Therapy for Visceral Hypersensitivity.Front Neurosci. 2021 Sep 28;15:696843. doi: 10.3389/fnins.2021.696843. eCollection 2021. Front Neurosci. 2021. PMID: 34658755 Free PMC article. Review.
-
Lesioning of TRPV1 expressing primary afferent neurons prevents PAR-2 induced motility, but not mechanical hypersensitivity in the rat colon.Neurogastroenterol Motil. 2012 Mar;24(3):e125-35. doi: 10.1111/j.1365-2982.2011.01848.x. Epub 2011 Dec 13. Neurogastroenterol Motil. 2012. PMID: 22168801 Free PMC article.
-
Mechanisms of Visceral Organ Crosstalk: Importance of Alterations in Permeability in Rodent Models.J Urol. 2015 Sep;194(3):804-11. doi: 10.1016/j.juro.2015.02.2944. Epub 2015 Mar 14. J Urol. 2015. PMID: 25776913 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

