Targeting sequences of UBXD8 and AAM-B reveal that the ER has a direct role in the emergence and regression of lipid droplets
- PMID: 19773358
- PMCID: PMC2758803
- DOI: 10.1242/jcs.054700
Targeting sequences of UBXD8 and AAM-B reveal that the ER has a direct role in the emergence and regression of lipid droplets
Abstract
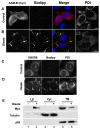
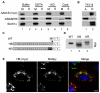
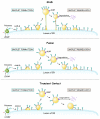
Lipid droplets are sites of neutral lipid storage thought to be actively involved in lipid homeostasis. A popular model proposes that droplets are formed in the endoplasmic reticulum (ER) by a process that begins with the deposition of neutral lipids between the membrane bilayer. As the droplet grows, it becomes surrounded by a monolayer of phospholipid derived from the outer half of the ER membrane, which contains integral membrane proteins anchored by hydrophobic regions. This model predicts that for an integral droplet protein inserted into the outer half of the ER membrane to reach the forming droplet, it must migrate in the plane of the membrane to sites of lipid accumulation. Here, we report the results of experiments that directly test this hypothesis. Using two integral droplet proteins that contain unique hydrophobic targeting sequences (AAM-B and UBXD8), we present evidence that both proteins migrate from their site of insertion in the ER to droplets that are forming in response to fatty acid supplementation. Migration to droplets occurs even when further protein synthesis is inhibited or dominant-negative Sar1 blocks transport to the Golgi complex. Surprisingly, when droplets are induced to disappear from the cell, both proteins return to the ER as the level of neutral lipid declines. These data suggest that integral droplet proteins form from and regress to the ER as part of a cyclic process that does not involve traffic through the secretory pathway.
Figures



References
-
- Altan-Bonnet, N., Sougrat, R. and Lippincott-Schwartz, J. (2004). Molecular basis for Golgi maintenance and biogenesis. Curr. Opin. Cell Biol. 16, 364-372. - PubMed
-
- Bartz, R., Li, W. H., Venables, B., Zehmer, J. K., Roth, M. R., Welti, R., Anderson, R. G., Liu, P. and Chapman, K. D. (2007a). Lipidomics reveals that adiposomes store ether lipids and mediate phospholipid traffic. J. Lipid Res. 48, 837-847. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

