Psychometric functions for ternary odor mixtures and their unmixed components
- PMID: 19773409
- PMCID: PMC2762054
- DOI: 10.1093/chemse/bjp062
Psychometric functions for ternary odor mixtures and their unmixed components
Abstract
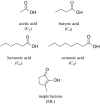
People are often able to reliably detect a mixture of 2 or more odorants, even if they cannot reliably detect the individual mixture components when presented individually. This phenomenon has been called mixture agonism. However, for some mixtures, agonism among mixture components is greater in barely detectable mixtures than in more easily detectable mixtures (level dependence). Most studies that have used rigorous methods have focused on simple, 2-component (binary) mixtures. The current work takes the next logical step to study detection of 3-component (ternary) mixtures. Psychometric functions were measured for 5 unmixed compounds and for 3 ternary mixtures of these compounds (2 of 5, forced-choice method). Experimenters used air dilution olfactometry to precisely control the duration and concentration of stimuli and used gas chromatography/mass spectrometry to verify vapor-phase concentrations. For 2 of the 3 mixtures, agonism was approximately additive in general agreement with similar work on binary mixtures. A third mixture was no more detectable than the most detectable component, demonstrating a lack of agonism. None of the 3 mixtures showed evidence of level dependence. Agonism may be common in ternary mixtures, but general rules of mixture interaction have yet to emerge. For now, detection of any mixture must be measured empirically.
Figures
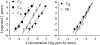
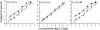
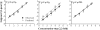
Similar articles
-
Odor detection of mixtures of homologous carboxylic acids and coffee aroma compounds by humans.J Agric Food Chem. 2009 Nov 11;57(21):9895-901. doi: 10.1021/jf901453r. J Agric Food Chem. 2009. PMID: 19817417
-
Human odor detection of homologous carboxylic acids and their binary mixtures.Chem Senses. 2007 Jun;32(5):475-82. doi: 10.1093/chemse/bjm016. Epub 2007 May 7. Chem Senses. 2007. PMID: 17488748
-
Perceptual blending in odor mixtures depends on the nature of odorants and human olfactory expertise.Chem Senses. 2012 Feb;37(2):159-66. doi: 10.1093/chemse/bjr086. Epub 2011 Aug 26. Chem Senses. 2012. PMID: 21873604
-
Superior Identification of Component Odors in a Mixture Is Linked to Autistic Traits in Children and Adults.Chem Senses. 2020 May 29;45(5):391-399. doi: 10.1093/chemse/bjaa026. Chem Senses. 2020. PMID: 32249289
-
The perception of odor is not a surrogate marker for chemical exposure: a review of factors influencing human odor perception.Clin Toxicol (Phila). 2013 Feb;51(2):70-6. doi: 10.3109/15563650.2013.767908. Clin Toxicol (Phila). 2013. PMID: 23387344 Review.
Cited by
-
Research on odor interaction between aldehyde compounds via a partial differential equation (PDE) model.Sensors (Basel). 2015 Jan 28;15(2):2888-901. doi: 10.3390/s150202888. Sensors (Basel). 2015. PMID: 25635413 Free PMC article.
-
Structure-activity relationships on the odor detectability of homologous carboxylic acids by humans.Exp Brain Res. 2010 Nov;207(1-2):75-84. doi: 10.1007/s00221-010-2430-0. Epub 2010 Oct 8. Exp Brain Res. 2010. PMID: 20931179 Free PMC article.
-
The perception of odor objects in everyday life: a review on the processing of odor mixtures.Front Psychol. 2014 Jun 2;5:504. doi: 10.3389/fpsyg.2014.00504. eCollection 2014. Front Psychol. 2014. PMID: 24917831 Free PMC article. Review.
References
-
- Araneda RC, Kini AD, Firestein S. The molecular receptive range of an odorant receptor. Nat Neurosci. 2000;3:1248–1255. - PubMed
-
- Baker RA. Odor effects of aqueous mixtures of organic chemicals. J Water Pollut Control. 1963;35:728–741.
-
- Berglund B, Berglund T, Lindvall T, Svensson LT. A quantitative principle of perceived intensity summation in odor mixtures. J Exp Psychol. 1973;100:29–38. - PubMed
-
- Brodin M, Laska M, Olsson MJ. Odor interaction between Bourgeonal and its antagonist undecanal. Chem Senses. 2009 doi: 10.1093/chemse/bjp044. - PubMed
-
- Brossard C, Rousseau F, Dumont J-P. Perceptual interactions between characteristic notes smelled above aqueous solutions of odorant mixtures. Chem Senses. 2007;32:319–327. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

