MicroRNA-34a inhibits glioblastoma growth by targeting multiple oncogenes
- PMID: 19773441
- PMCID: PMC2756313
- DOI: 10.1158/0008-5472.CAN-09-0529
MicroRNA-34a inhibits glioblastoma growth by targeting multiple oncogenes
Abstract
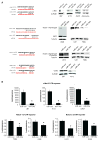
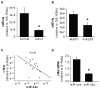
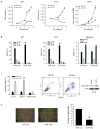
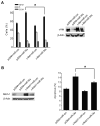
MicroRNA-34a (miR-34a) is a transcriptional target of p53 that is down-regulated in some cancer cell lines. We studied the expression, targets, and functional effects of miR-34a in brain tumor cells and human gliomas. Transfection of miR-34a down-regulated c-Met in human glioma and medulloblastoma cells and Notch-1, Notch-2, and CDK6 protein expressions in glioma cells. miR-34a expression inhibited c-Met reporter activities in glioma and medulloblastoma cells and Notch-1 and Notch-2 3'-untranslated region reporter activities in glioma cells and stem cells. Analysis of human specimens showed that miR-34a expression is down-regulated in glioblastoma tissues as compared with normal brain and in mutant p53 gliomas as compared with wild-type p53 gliomas. miR-34a levels in human gliomas inversely correlated to c-Met levels measured in the same tumors. Transient transfection of miR-34a into glioma and medulloblastoma cell lines strongly inhibited cell proliferation, cell cycle progression, cell survival, and cell invasion, but transfection of miR-34a into human astrocytes did not affect cell survival and cell cycle status. Forced expression of c-Met or Notch-1/Notch-2 transcripts lacking the 3'-untranslated region sequences partially reversed the effects of miR-34a on cell cycle arrest and cell death in glioma cells and stem cells, respectively. Also, transient expression of miR-34a in glioblastoma cells strongly inhibited in vivo glioma xenograft growth. Together, these findings represent the first comprehensive analysis of the role of miR-34a in gliomas. They show that miR-34a suppresses brain tumor growth by targeting c-Met and Notch. The results also suggest that miR-34a could serve as a potential therapeutic agent for brain tumors.
Figures

Similar articles
-
microRNA-34a is tumor suppressive in brain tumors and glioma stem cells.Cell Cycle. 2010 Mar 15;9(6):1031-6. doi: 10.4161/cc.9.6.10987. Epub 2010 Mar 15. Cell Cycle. 2010. PMID: 20190569 Free PMC article.
-
P53-induced microRNA-107 inhibits proliferation of glioma cells and down-regulates the expression of CDK6 and Notch-2.Neurosci Lett. 2013 Feb 8;534:327-32. doi: 10.1016/j.neulet.2012.11.047. Epub 2012 Dec 7. Neurosci Lett. 2013. PMID: 23220650
-
MicroRNA-34a targets notch1 and inhibits cell proliferation in glioblastoma multiforme.Cancer Biol Ther. 2011 Sep 15;12(6):477-83. doi: 10.4161/cbt.12.6.16300. Epub 2011 Sep 15. Cancer Biol Ther. 2011. PMID: 21743299
-
Targeting strategies on miRNA-21 and PDCD4 for glioblastoma.Arch Biochem Biophys. 2015 Aug 15;580:64-74. doi: 10.1016/j.abb.2015.07.001. Epub 2015 Jul 2. Arch Biochem Biophys. 2015. PMID: 26142886 Review.
-
The elephant in the room: do microRNA-based therapies have a realistic chance of succeeding for brain tumors such as glioblastoma?J Neurooncol. 2011 Jul;103(3):429-36. doi: 10.1007/s11060-010-0449-5. Epub 2010 Nov 17. J Neurooncol. 2011. PMID: 21082214 Free PMC article. Review.
Cited by
-
Epigenetic alterations involved in cancer stem cell reprogramming.Mol Oncol. 2012 Dec;6(6):620-36. doi: 10.1016/j.molonc.2012.10.006. Epub 2012 Oct 26. Mol Oncol. 2012. PMID: 23141800 Free PMC article. Review.
-
Non-coding RNAs as therapeutic targets in cancer and its clinical application.J Pharm Anal. 2024 Jul;14(7):100947. doi: 10.1016/j.jpha.2024.02.001. Epub 2024 Feb 8. J Pharm Anal. 2024. PMID: 39149142 Free PMC article. Review.
-
Dual biomarkers long non-coding RNA GAS5 and microRNA-34a co-expression signature in common solid tumors.PLoS One. 2018 Oct 5;13(10):e0198231. doi: 10.1371/journal.pone.0198231. eCollection 2018. PLoS One. 2018. PMID: 30289954 Free PMC article.
-
The microRNA and messengerRNA profile of the RNA-induced silencing complex in human primary astrocyte and astrocytoma cells.PLoS One. 2010 Oct 18;5(10):e13445. doi: 10.1371/journal.pone.0013445. PLoS One. 2010. PMID: 20976148 Free PMC article.
-
Multiple receptor tyrosine kinases converge on microRNA-134 to control KRAS, STAT5B, and glioblastoma.Cell Death Differ. 2014 May;21(5):720-34. doi: 10.1038/cdd.2013.196. Epub 2014 Jan 17. Cell Death Differ. 2014. PMID: 24440911 Free PMC article.
References
-
- Li Y, Lal B, Kwon S, et al. The scatter factor/hepatocyte growth factor: c-met pathway in human embryonal central nervous system tumor malignancy. Cancer Res. 2005;65:9355–62. - PubMed
-
- Laterra J, Nam M, Rosen E, Rao JS, Johnston P. Scatter factor/hepatocyte growth factor gene transfer to 9L glioma cells enhances glioma growth and angiogenesis in vivo. Lab Invest. 1997;76:565–77. - PubMed
-
- Abounader R, Ranganathan S, Lal B, et al. Reversion of human glioblastoma malignancy by U1 small nuclear RNA/ribozyme targeting of scatter factor/hepatocyte growth factor and c- met expression. J Natl Cancer Inst. 1999;91:1548–56. - PubMed
-
- Rosen EM, Laterra J, Joseph A, et al. Scatter factor expression and regulation in human glial tumors. Intl J Cancer. 1996;67:248–55. - PubMed
-
- Moriyama T, Kataoka H, Kawano Y, et al. Comparative analysis of expression of hepatocyte growth factor and its receptor, c-met, in gliomas, meningiomas and schwannomas. Canc Lett. 1998;124:149–55. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

