Molecular distinctions between stasis and telomere attrition senescence barriers shown by long-term culture of normal human mammary epithelial cells
- PMID: 19773443
- PMCID: PMC2782785
- DOI: 10.1158/0008-5472.CAN-09-0270
Molecular distinctions between stasis and telomere attrition senescence barriers shown by long-term culture of normal human mammary epithelial cells
Abstract
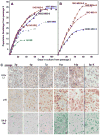
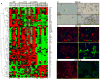
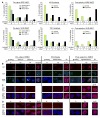
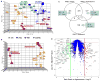
Normal human epithelial cells in culture have generally shown a limited proliferative potential of approximately 10 to 40 population doublings before encountering a stress-associated senescence barrier (stasis) associated with elevated levels of cyclin-dependent kinase inhibitors p16 and/or p21. We now show that simple changes in medium composition can expand the proliferative potential of human mammary epithelial cells (HMEC) initiated as primary cultures to 50 to 60 population doublings followed by p16-positive, senescence-associated beta-galactosidase-positive stasis. We compared the properties of growing and senescent pre-stasis HMEC with growing and senescent post-selection HMEC, that is, cells grown in a serum-free medium that overcame stasis via silencing of p16 expression and that display senescence associated with telomere dysfunction. Cultured pre-stasis populations contained cells expressing markers associated with luminal and myoepithelial HMEC lineages in vivo in contrast to the basal-like phenotype of the post-selection HMEC. Gene transcript and protein expression, DNA damage-associated markers, mean telomere restriction fragment length, and genomic stability differed significantly between HMEC populations at the stasis versus telomere dysfunction senescence barriers. Senescent isogenic fibroblasts showed greater similarity to HMEC at stasis than at telomere dysfunction, although their gene transcript profile was distinct from HMEC at both senescence barriers. These studies support our model of the senescence barriers encountered by cultured HMEC in which the first barrier, stasis, is retinoblastoma-mediated and independent of telomere length, whereas a second barrier (agonescence or crisis) results from telomere attrition leading to telomere dysfunction. Additionally, the ability to maintain long-term growth of genomically stable multilineage pre-stasis HMEC populations can greatly enhance experimentation with normal HMEC.
Figures

References
-
- Stampfer MR. Cholera toxin stimulation of human mammary epithelial cells in culture. In Vitro. 1982;18:531–7. - PubMed
-
- Romanov S, Kozakiewicz K, Holst C, Stampfer MR, Haupt LM, Tlsty T. Normal human mammary epithelial cells spontaneously escape senescence and acquire genomic changes. Nature. 2001;409:633–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

