Heme oxygenase-2 deletion causes endothelial cell activation marked by oxidative stress, inflammation, and angiogenesis
- PMID: 19773531
- PMCID: PMC2784722
- DOI: 10.1124/jpet.109.158352
Heme oxygenase-2 deletion causes endothelial cell activation marked by oxidative stress, inflammation, and angiogenesis
Abstract
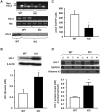
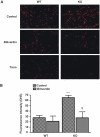
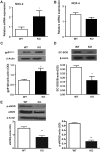
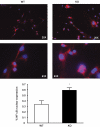
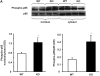
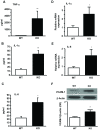
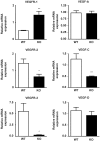
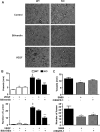
In previous studies, we have shown that heme oxygenase (HO)-2 null [HO-2(-/-)] mice exhibit a faulty response to injury; chronic inflammation and massive neovascularization replaced resolution of inflammation and tissue repair. Endothelial cells play an active and essential role in the control of inflammation and the process of angiogenesis. We examined whether HO-2 deletion affects endothelial cell function. Under basal conditions, HO-2(-/-) aortic endothelial cells (mAEC) showed a 3-fold higher expression of vascular endothelial growth factor receptor 1 and a marked angiogenic response compared with wild-type (WT) cells. Compared with WT cells, HO-2(-/-) mAEC showed a 2-fold reduction in HO activity and marked increases in levels of gp91(phox)/NADPH oxidase isoform, superoxide, nuclear factor kappaB activation, and expression of inflammatory cytokines, including interleukin (IL)-1alpha and IL-6. HO-2 deletion transforms endothelial cells from a "normal" to an "activated" phenotype characterized by increases in inflammatory, oxidative, and angiogenic factors. This switch may be the result of reduced HO activity and the associated reduction in the cytoprotective HO products, carbon monoxide and biliverdin/bilirubin, because addition of biliverdin to HO-2(-/-) cells attenuated angiogenesis and reduced superoxide production. This transformation underscores the importance of HO-2 in the regulation of endothelial cell homeostasis.
Figures
Similar articles
-
Bilirubin from heme oxygenase-1 attenuates vascular endothelial activation and dysfunction.Arterioscler Thromb Vasc Biol. 2005 Jan;25(1):155-60. doi: 10.1161/01.ATV.0000148405.18071.6a. Epub 2004 Oct 21. Arterioscler Thromb Vasc Biol. 2005. PMID: 15499042
-
HO-2 provides endogenous protection against oxidative stress and apoptosis caused by TNF-alpha in cerebral vascular endothelial cells.Am J Physiol Cell Physiol. 2006 Nov;291(5):C897-908. doi: 10.1152/ajpcell.00032.2006. Epub 2006 Jul 5. Am J Physiol Cell Physiol. 2006. PMID: 16822952
-
Glutamate induces oxidative stress and apoptosis in cerebral vascular endothelial cells: contributions of HO-1 and HO-2 to cytoprotection.Am J Physiol Cell Physiol. 2006 May;290(5):C1399-410. doi: 10.1152/ajpcell.00386.2005. Epub 2005 Dec 21. Am J Physiol Cell Physiol. 2006. PMID: 16371440
-
Cerebroprotective functions of HO-2.Curr Pharm Des. 2008;14(5):443-53. doi: 10.2174/138161208783597380. Curr Pharm Des. 2008. PMID: 18289071 Free PMC article. Review.
-
Protective role of heme oxygenase in the blood vessel wall during atherogenesis.Biochem Cell Biol. 2004 Jun;82(3):351-9. doi: 10.1139/o04-006. Biochem Cell Biol. 2004. PMID: 15181468 Review.
Cited by
-
The impact of heme oxygenase-2 on pharmacological research: A bibliometric analysis and beyond.Front Pharmacol. 2023 Apr 19;14:1156333. doi: 10.3389/fphar.2023.1156333. eCollection 2023. Front Pharmacol. 2023. PMID: 37153762 Free PMC article.
-
Dopamine Receptors and the Kidney: An Overview of Health- and Pharmacological-Targeted Implications.Biomolecules. 2021 Feb 10;11(2):254. doi: 10.3390/biom11020254. Biomolecules. 2021. PMID: 33578816 Free PMC article. Review.
-
Heme Oxygenase 1 as a Therapeutic Target in Acute Kidney Injury.Am J Kidney Dis. 2017 Apr;69(4):531-545. doi: 10.1053/j.ajkd.2016.10.037. Epub 2017 Jan 27. Am J Kidney Dis. 2017. PMID: 28139396 Free PMC article. Review.
-
Thiol/Disulfide redox switches in the regulation of heme binding to proteins.Antioxid Redox Signal. 2011 Mar 15;14(6):1039-47. doi: 10.1089/ars.2010.3436. Epub 2010 Dec 27. Antioxid Redox Signal. 2011. PMID: 20812781 Free PMC article. Review.
-
The manganese(III) porphyrin MnTnHex-2-PyP5+ modulates intracellular ROS and breast cancer cell migration: Impact on doxorubicin-treated cells.Redox Biol. 2019 Jan;20:367-378. doi: 10.1016/j.redox.2018.10.016. Epub 2018 Oct 25. Redox Biol. 2019. PMID: 30408752 Free PMC article.
References
-
- Abraham NG, Kappas A. (2008) Pharmacological and clinical aspects of heme oxygenase. Pharmacol Rev 60:79–127 - PubMed
-
- Ago T, Kitazono T, Ooboshi H, Iyama T, Han YH, Takada J, Wakisaka M, Ibayashi S, Utsumi H, Iida M. (2004) Nox4 as the major catalytic component of an endothelial NAD(P)H oxidase. Circulation 109:227–233 - PubMed
-
- Bradford MM. (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254 - PubMed
-
- Bussolati B, Ahmed A, Pemberton H, Landis RC, Di Carlo F, Haskard DO, Mason JC. (2004) Bifunctional role for VEGF-induced heme oxygenase-1 in vivo: induction of angiogenesis and inhibition of leukocytic infiltration. Blood 103:761–766 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

