The fifth transmembrane domain of angiotensin II Type 1 receptor participates in the formation of the ligand-binding pocket and undergoes a counterclockwise rotation upon receptor activation
- PMID: 19773549
- PMCID: PMC2797267
- DOI: 10.1074/jbc.M109.051839
The fifth transmembrane domain of angiotensin II Type 1 receptor participates in the formation of the ligand-binding pocket and undergoes a counterclockwise rotation upon receptor activation
Abstract
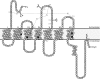
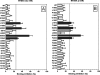
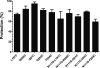
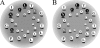
The octapeptide hormone angiotensin II exerts a wide variety of cardiovascular effects through the activation of the angiotensin II Type 1 (AT(1)) receptor, which belongs to the G protein-coupled receptor superfamily. Like other G protein- coupled receptors, the AT(1) receptor possesses seven transmembrane domains that provide structural support for the formation of the ligand-binding pocket. The role of the fifth transmembrane domain (TMD5) was investigated using the substituted cysteine accessibility method. All of the residues within Thr-190 to Leu-217 region were mutated one at a time to cysteine, and after expression in COS-7 cells, the mutant receptors were treated with the sulfhydryl-specific alkylating agent methanethiosulfonate-ethylammonium (MTSEA). MTSEA reacts selectively with water-accessible, free sulfhydryl groups of endogenous or introduced point mutation cysteines. If a cysteine is found in the binding pocket, the covalent modification will affect the binding kinetics of the ligand. MTSEA substantially decreased the binding affinity of L197C-AT(1), N200C-AT(1), I201C-AT(1), G203C-AT(1), and F204C-AT(1) mutant receptors, which suggests that these residues orient themselves within the water-accessible binding pocket of the AT(1) receptor. Interestingly, this pattern of acquired MTSEA sensitivity was altered for TMD5 reporter cysteines engineered in a constitutively active N111G-AT(1) receptor background. Indeed, mutant I201C-N111G-AT(1) became more sensitive to MTSEA, whereas mutant G203C-N111G-AT(1) lost some sensitivity. Our results suggest that constitutive activation of AT(1) receptor causes an apparent counterclockwise rotation of TMD5 as viewed from the extracellular side.
Figures

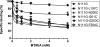

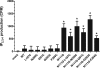
References
-
- de Gasparo M., Catt K. J., Inagami T., Wright J. W., Unger T. (2000) Pharmacol. Rev. 52, 415–472 - PubMed
-
- Burnier M. (2001) Circulation 103, 904–912 - PubMed
-
- Miura S., Saku K., Karnik S. S. (2003) Hypertens. Res. 26, 937–943 - PubMed
-
- Kojima I., Kojima K., Kreutter D., Rasmussen H. (1984) J. Biol. Chem. 259, 14448–14457 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

