Weekly administration of sagopilone (ZK-EPO), a fully synthetic epothilone, in patients with refractory solid tumours: results of a phase I trial
- PMID: 19773753
- PMCID: PMC2768435
- DOI: 10.1038/sj.bjc.6605327
Weekly administration of sagopilone (ZK-EPO), a fully synthetic epothilone, in patients with refractory solid tumours: results of a phase I trial
Abstract
Background: Epothilones are a novel class of microtubule-stabilising agents, and sagopilone is a fully synthetic epothilone that has shown marked in vivo and in vitro preclinical activity.
Methods: This phase I, open-label study investigated the maximum tolerated dose (MTD) and dose-limiting toxicities (DLTs) of weekly sagopilone. Twenty-three patients with malignancy resistant or refractory to standard treatment were enrolled into this study evaluating sagopilone doses from 0.6 to 7.0 mg m(-2).
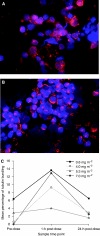
Results: The incidence of drug-related haematological adverse events (AEs) was low, with two grade 3 events observed. Nonhaematological AEs were generally mild and reversible; increased gamma-GT was the only grade 4 event and grade 3 events comprised peripheral neuropathy (n=2), diarrhoea (n=1) and fatigue (n=1). Two grade 3 events were DLTs (diarrhoea and peripheral neuropathy at 7.0 mg m(-2)). The MTD of weekly sagopilone was therefore established as 5.3 mg m(-2). Stable disease was the best overall response (n=3). Microtubule bundle formation in peripheral blood mononuclear cells increased post-treatment, peaking after 1 h. Sagopilone disposition was similar across treatment courses and showed rapidly decreasing serum concentrations after infusion end and a long terminal disposition phase with no obvious accumulation in the serum, probably reflecting a fast uptake into tissues followed by a slow release.
Conclusion: Weekly administration of sagopilone could represent an alternative to the 3-weekly administration currently evaluated in phase II trials.
Figures

Similar articles
-
First clinical pharmacokinetic dose-escalation study of sagopilone, a novel, fully synthetic epothilone, in Japanese patients with refractory solid tumors.Invest New Drugs. 2012 Dec;30(6):2327-33. doi: 10.1007/s10637-011-9773-7. Epub 2011 Dec 4. Invest New Drugs. 2012. PMID: 22139065 Clinical Trial.
-
Phase I study of the novel, fully synthetic epothilone sagopilone (ZK-EPO) in patients with solid tumors.Ann Oncol. 2010 Mar;21(3):633-639. doi: 10.1093/annonc/mdp491. Epub 2009 Oct 30. Ann Oncol. 2010. PMID: 19880436 Clinical Trial.
-
A phase II trial evaluating two schedules of sagopilone (ZK-EPO), a novel epothilone, in patients with platinum-resistant ovarian cancer.Ann Oncol. 2011 Nov;22(11):2411-2416. doi: 10.1093/annonc/mdq780. Epub 2011 Mar 3. Ann Oncol. 2011. PMID: 21372124 Clinical Trial.
-
Sagopilone, a microtubule stabilizer for the potential treatment of cancer.Curr Opin Investig Drugs. 2009 Dec;10(12):1359-71. Curr Opin Investig Drugs. 2009. PMID: 19943207 Review.
-
Sagopilone (ZK-EPO): from a natural product to a fully synthetic clinical development candidate.Expert Opin Investig Drugs. 2008 Nov;17(11):1735-48. doi: 10.1517/13543784.17.11.1735. Expert Opin Investig Drugs. 2008. PMID: 18922109 Review.
Cited by
-
Microtubule-binding agents: a dynamic field of cancer therapeutics.Nat Rev Drug Discov. 2010 Oct;9(10):790-803. doi: 10.1038/nrd3253. Nat Rev Drug Discov. 2010. PMID: 20885410 Free PMC article. Review.
-
Phase I dose escalation study of KOS-1584, a novel epothilone, in patients with advanced solid tumors.Cancer Chemother Pharmacol. 2012 Feb;69(2):523-31. doi: 10.1007/s00280-011-1724-7. Epub 2011 Aug 27. Cancer Chemother Pharmacol. 2012. PMID: 21874318 Free PMC article. Clinical Trial.
-
Phase I clinical, pharmacokinetic, and pharmacodynamic study of KOS-862 (Epothilone D) in patients with advanced solid tumors and lymphoma.Invest New Drugs. 2012 Dec;30(6):2294-302. doi: 10.1007/s10637-011-9765-7. Epub 2011 Nov 10. Invest New Drugs. 2012. PMID: 22072399 Free PMC article. Clinical Trial.
-
First clinical pharmacokinetic dose-escalation study of sagopilone, a novel, fully synthetic epothilone, in Japanese patients with refractory solid tumors.Invest New Drugs. 2012 Dec;30(6):2327-33. doi: 10.1007/s10637-011-9773-7. Epub 2011 Dec 4. Invest New Drugs. 2012. PMID: 22139065 Clinical Trial.
-
Phase I/II study of sagopilone (ZK-EPO) plus carboplatin in women with recurrent platinum-sensitive ovarian cancer.Br J Cancer. 2012 Jan 3;106(1):70-6. doi: 10.1038/bjc.2011.499. Epub 2011 Nov 22. Br J Cancer. 2012. PMID: 22108514 Free PMC article. Clinical Trial.
References
-
- Goldspiel BR (1997) Clinical overview of the taxanes. Pharmacotherapy 17: 110S–125S - PubMed
-
- Graff J, Smith DC, Neerukonda L, Alonso M, Edgar A, Wang Y, Beer TM, The Prostate Cancer Clinical Trials Consortium (2008) Phase II study of sagopilone (ZK-EPO) plus prednisone as first-line chemotherapy in patients with metastatic androgen-independent prostate cancer 44th ASCO Annual Meeting, Chicago, Illinois, USA, May 30–June 3, Poster
-
- Hoffmann J, Vitale I, Buchmann B, Galluzzi L, Schwede W, Senovilla L, Skuballa W, Vivet S, Lichtner RB, Vicencio JM, Panaretakis T, Siemeister G, Lage H, Nanty L, Hammer S, Mittelstaedt K, Winsel S, Eschenbrenner J, Castedo M, Demarche C, Klar U, Kroemer G (2008) Improved cellular pharmacokinetics and pharmacodynamics underlie the wide anticancer activity of sagopilone. Cancer Res 68: 5301–5308 - PubMed
-
- Horwitz SB, Cohen D, Rao S, Ringel I, Shen H-J, Yang C-PH (1993) Taxol: mechanisms of action and resistance. J Natl Cancer Inst Monogr 15: 55–61 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

