Phase II study of cetuximab in combination with cisplatin and docetaxel in patients with untreated advanced gastric or gastro-oesophageal junction adenocarcinoma (DOCETUX study)
- PMID: 19773760
- PMCID: PMC2768436
- DOI: 10.1038/sj.bjc.6605319
Phase II study of cetuximab in combination with cisplatin and docetaxel in patients with untreated advanced gastric or gastro-oesophageal junction adenocarcinoma (DOCETUX study)
Abstract
Background: The conventional treatment options for advanced gastric patients remain unsatisfactory in terms of response rate, response duration, toxicity, and overall survival benefit. The purpose of this phase II study was to evaluate the activity and safety of cetuximab combined with cisplatin and docetaxel as a first-line treatment for advanced gastric or gastro-oesophageal junction adenocarcinoma.
Methods: Untreated patients with histologically confirmed advanced gastric or gastro-oesophageal adenocarcinoma received cetuximab at an initial dose of 400 mg m(-2) i.v. followed by weekly doses of 250 mg m(-2), cisplatin 75 mg m(-2) i.v. on day 1, docetaxel 75 mg m(-2) i.v. on day 1, every 3 weeks, for a maximum of 6 cycles, and then cetuximab maintenance treatment was allowed in patients with a complete response, partial response, or stable disease.
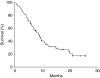
Results: Seventy-two patients (stomach 81.9% and gastro-oesophageal junction 18.1%; locally advanced disease 4.2%; and metastatic disease 95.8%) were enrolled. The ORR was 41.2% (95% CI, 29.5-52.9). Median time to progression was 5 months (95% CI, 3.7-5.4). Median survival time was 9 months (95% CI, 7-11). The most frequent grades 3-4 toxicity was neutropenia (44.4%). No toxic death was observed.
Conclusions: The addition of cetuximab to the cisplatin/docetaxel regimen improved the ORR of the cisplatin/docetaxel doublet in the first-line treatment of advanced gastric and gastro-oesophageal junction adenocarcinoma, but this combination did not improve the TTP and OS. The toxicity of cisplatin/docetaxel chemotherapy was not affected by the addition of cetuximab.
Figures
References
-
- Ajani JA, Fodor MB, Tjulandin SA, Moiseyenko VM, Chao Y, Cabral Filho S, Majlis A, Assadourian S, Van Cutsem E (2005) Phase II multi-institutional randomized trial of docetaxel plus cisplatin with or without fluorouracil in patients with untreated, advanced gastric, or gastroesophageal adenocarcinoma. J Clin Oncol 23: 5660–5667 - PubMed
-
- Ajani JA, Rodriguez W, Bodoky G, Moiseyenko V, Lichinitser M, Gorbunova V, Vynnychenko I, Garin A, Lang I, Falcon S (2009) Multicenter phase III comparison of cisplatin/S-1 (CS) with cisplatin/5-FU (CF) as first-line therapy in patients with advanced gastric cancer (FLAGS). Proc ASCO GI, Abstract 8
-
- Albanell J, Rojo F, Baselga J (2001) Pharmacodynamic studies with the epidermal growth factor receptor tyrosine kinase inhibitor ZD 1839. Semin Oncol 28(Suppl 16): 56–66 - PubMed
-
- Bokemeyer C, Bondarenko I, Makhson A, Hartmann JT, Aparicio J, de Braud F, Donea S, Ludwig H, Schuch G, Stroh C, Loos AH, Zubel A, Koralewski P (2009) Fluorouracil, leucovorin, and oxaliplatin with and without cetuximab in the first-line treatment of metastatic colorectal cancer. J Clin Oncol 10: 663–671 - PubMed
-
- Bosetti C, Bertuccio P, Levi F, Lucchini F, Negri E, La Vecchia C (2008) Cancer mortality in the European Union, 1970–2003, with a joinpoint analysis. Ann Oncol 19: 631–640 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical