p38 MAPK activity is stimulated by vascular endothelial growth factor receptor 2 activation and is essential for shear stress-induced angiogenesis
- PMID: 19774558
- PMCID: PMC3947629
- DOI: 10.1002/jcp.21924
p38 MAPK activity is stimulated by vascular endothelial growth factor receptor 2 activation and is essential for shear stress-induced angiogenesis
Abstract
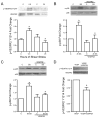
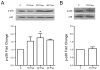
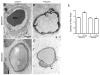
Increased capillary shear stress induces angiogenesis in skeletal muscle, but the signaling mechanisms underlying this response are not known. We hypothesize that shear stress-dependent activation of vascular endothelial growth factor receptor 2 (VEGFR2) causes p38 and ERK1/2 phosphorylation, which contribute to shear stress-induced angiogenesis. Skeletal muscle microvascular endothelial cells were sheared (12 dynes/cm(2), 0.5-24 h). VEGFR2-Y1214 phosphorylation increased in response to elevated shear stress and VEGF stimulation. p38 and ERK1/2 phosphorylation increased at 2 h of shear stress but only p38 remained phosphorylated at 6 and 24 h of shear stress. VEGFR2 inhibition abrogated p38, but not ERK1/2 phosphorylation. VEGF production was increased in response to shear stress at 6 h, and this increased production was abolished by p38 inhibition. Male Sprague-Dawley rats were administered prazosin (50 mg/L drinking water, 1, 2, 4, or 7 days) to induce chronically elevated capillary shear stress in skeletal muscle. In some experiments, mini-osmotic pumps were used to dispense p38 inhibitor SB203580 or its inactive analog SB202474, to the extensor digitorum longus (EDL) of control and prazosin-treated rats. Immunostaining and Western blotting showed increases in p38 phosphorylation in capillaries from rats treated with prazosin for 2 days but returned to basal levels at 4 and 7 days. p38 inhibition abolished the increase in capillary to muscle fiber ratio seen after 7 days of prazosin treatment. Our data suggest that p38 activation is necessary for shear stress-dependent angiogenesis.
Figures





References
-
- Baum O, Da Silva-Azevedo L, Willerding G, Wockel A, Planitzer G, Gossrau R, Pries AR, Zakrzewicz A. Endothelial NOS is main mediator for shear stress-dependent angiogenesis in skeletal muscle after prazosin administration. Am J Physiol Heart Circ Physiol. 2004;287:H2300–8. - PubMed
-
- Boyd PJ, Doyle J, Gee E, Pallan S, Haas TL. MAPK signaling regulates endothelial cell assembly into networks and expression of MT1-MMP and MMP-2. Am J Physiol Cell Physiol. 2005;288:C659–68. - PubMed
-
- Chen KD, Li YS, Kim M, Li S, Yuan S, Chien S, Shyy JY. Mechanotransduction in response to shear stress. Roles of receptor tyrosine kinases, integrins, and Shc. J Biol Chem. 1999;274:18393–18400. - PubMed
-
- Chien S. Mechanotransduction and endothelial cell homeostasis: the wisdom of the cell. Am J Physiol Heart Circ Physiol. 2007;292:H1209–24. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

