Human embryonic stem cells are pre-mitotically committed to self-renewal and acquire a lengthened G1 phase upon lineage programming
- PMID: 19774559
- PMCID: PMC2804950
- DOI: 10.1002/jcp.21925
Human embryonic stem cells are pre-mitotically committed to self-renewal and acquire a lengthened G1 phase upon lineage programming
Abstract
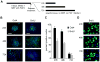
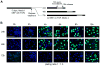
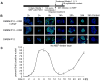
Self-renewal of human embryonic stem (hES) cells proceeds by a unique abbreviated cell cycle with a shortened G1 phase and distinctions in molecular cell cycle regulatory parameters. In this study, we show that early lineage-commitment of pluripotent hES cells modifies cell cycle kinetics. Human ES cells acquire a lengthened G1 within 72 h after lineage-programming is initiated, as reflected by loss of the pluripotency factor Oct4 and alterations in nuclear morphology. In hES cells that maintain the pristine pluripotent state, we find that autocrine mechanisms contribute to sustaining the abbreviated cell cycle. Our data show that naïve and mitotically synchronized pluripotent hES cells are competent to initiate two consecutive S phases in the absence of external growth factors. We conclude that short-term self-renewal of pluripotent hES cells occurs autonomously, in part due to secreted factors, and that pluripotency is functionally linked to the abbreviated hES cell cycle.
Figures



References
-
- Amit M, Carpenter MK, Inokuma MS, Chiu CP, Harris CP, Waknitz MA, Itskovitz-Eldor J, Thomson JA. Clonally derived human embryonic stem cell lines maintain pluripotency and proliferative potential for prolonged periods of culture. Dev Biol. 2000;227:271–278. - PubMed
-
- Becker KA, Ghule PN, Therrien JA, Lian JB, Stein JL, van Wijnen AJ, Stein GS. Self-renewal of human embryonic stem cells is supported by a shortened G1 cell cycle phase. J Cell Physiol. 2006;209:883–893. - PubMed
-
- Becker KA, Stein JL, Lian JB, van Wijnen AJ, Stein GS. Establishment of histone gene regulation and cell cycle checkpoint control in human embryonic stem cells. J Cell Physiol. 2007;210:517–526. - PubMed
-
- Blagosklonny MV, Pardee AB. The restriction point of the cell cycle. Cell Cycle. 2002;1:103–110. - PubMed
-
- Bodnar MS, Meneses JJ, Rodriguez RT, Firpo MT. Propagation and maintenance of undifferentiated human embryonic stem cells. Stem Cells Dev. 2004;13:243–253. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

