Total synthesis and stereochemical assignment of (-)-ushikulide A
- PMID: 19775093
- PMCID: PMC2791109
- DOI: 10.1021/ja906056v
Total synthesis and stereochemical assignment of (-)-ushikulide A
Abstract
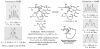
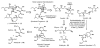
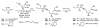
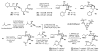
We report the determination of the full stereostructure of (-)-ushikulide A (1), a spiroketal containing macrolide by total synthesis. Ushikulide A (1) was isolated from a culture broth of Streptomyces sp. IUK-102 and exhibits potent immunosuppressant activity (IC(50) = 70 nM). To embark upon an ushikulide A synthesis, a tentative assignment was made based on analogy to cytovaricin (2), a related macrolide isolated from a culture of Streptomyces diastatochromogenes whose full structure was previously established via synthesis and X-ray crystallography. This report delineates studies on several key steps, namely a direct aldol reaction catalyzed by the dinuclear zinc ProPhenol complex, a metal catalyzed spiroketalization, as well as application of an unprecedented asymmetric alkynylation of a simple saturated aldehyde with methyl propiolate to prepare the nucleophilic partner for a Marshall-Tamaru propargylation. These studies culminated in the first total synthesis and stereochemical assignment of (-)-ushikulide A and significantly extended the scope of the above-mentioned methodologies.
Figures











Similar articles
-
Exploiting orthogonally reactive functionality: synthesis and stereochemical assignment of (-)-ushikulide A.J Am Chem Soc. 2008 Dec 3;130(48):16190-2. doi: 10.1021/ja807127s. J Am Chem Soc. 2008. PMID: 18989964 Free PMC article.
-
Structure of the new spiroketal-macrolide A82548A.J Antibiot (Tokyo). 1995 Sep;48(9):990-6. doi: 10.7164/antibiotics.48.990. J Antibiot (Tokyo). 1995. PMID: 7592067
-
Synthesis of a C(1)-C(23) fragment for spirastrellolide E: development of a mechanistic rationale for spiroketalization.Org Lett. 2015 Apr 17;17(8):1898-901. doi: 10.1021/acs.orglett.5b00595. Epub 2015 Apr 6. Org Lett. 2015. PMID: 25844543 Free PMC article.
-
Recent developments in transition metal-catalysed spiroketalisation.Org Biomol Chem. 2014 Oct 14;12(38):7423-32. doi: 10.1039/c4ob01325e. Org Biomol Chem. 2014. PMID: 25137016 Review.
-
Total synthesis of oxacyclic macrodiolide natural products.Chem Rev. 2005 Dec;105(12):4348-78. doi: 10.1021/cr040629a. Chem Rev. 2005. PMID: 16351047 Review. No abstract available.
Cited by
-
Development of Zn-ProPhenol-catalyzed asymmetric alkyne addition: synthesis of chiral propargylic alcohols.Chemistry. 2012 Dec 14;18(51):16498-509. doi: 10.1002/chem.201202085. Epub 2012 Oct 23. Chemistry. 2012. PMID: 23097281 Free PMC article.
-
Lewis Base Catalyzed, Sulfenium Ion Initiated Enantioselective, Spiroketalization Cascade.J Org Chem. 2021 Nov 5;86(21):14250-14289. doi: 10.1021/acs.joc.1c02271. Epub 2021 Oct 21. J Org Chem. 2021. PMID: 34672623 Free PMC article.
-
Gold-catalyzed glycosidation for the synthesis of trisaccharides by applying the armed-disarmed strategy.Beilstein J Org Chem. 2013 Oct 18;9:2147-55. doi: 10.3762/bjoc.9.252. eCollection 2013. Beilstein J Org Chem. 2013. PMID: 24204427 Free PMC article.
-
Development of the Synthesis of Desepoxy-Tedanolide C.J Org Chem. 2024 Feb 16;89(4):2408-2430. doi: 10.1021/acs.joc.3c02437. Epub 2024 Jan 25. J Org Chem. 2024. PMID: 38271689 Free PMC article.
-
The biosynthetic pathway to ossamycin, a macrocyclic polyketide bearing a spiroacetal moiety.PLoS One. 2019 Apr 30;14(4):e0215958. doi: 10.1371/journal.pone.0215958. eCollection 2019. PLoS One. 2019. PMID: 31039188 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

