Peptide targeting of platinum anti-cancer drugs
- PMID: 19775102
- PMCID: PMC2891414
- DOI: 10.1021/bc900065r
Peptide targeting of platinum anti-cancer drugs
Abstract
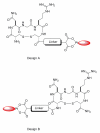
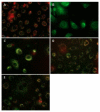
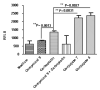
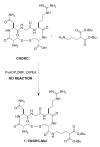
Besides various side effects caused by platinum anticancer drugs, they are not efficiently absorbed by the tumor cells. Two Pt-peptide conjugates; cyclic mPeg-CNGRC-Pt (7) and cyclic mPeg-CNGRC-Pten (8) bearing the Asn-Gly-Arg (NGR) targeting sequence, a malonoyl linker, and low molecular weight miniPEG groups have been synthesized. The platinum ligand was attached to the peptide via the carboxylic end of the malonate group at the end of the peptide. The pegylated peptide is nontoxic and highly soluble in water. Platinum conjugates synthesized using the pegylated peptides are also water-soluble with reduced or eliminated peptide immunogenicity. The choice of carboplatin as our untargeted platinum complex was due to the fact that the malonate linker chelates platinum in a manner similar to that of carboplatin. Cell toxicity assay and competition assay on the PC-3 cells (CD13 positive receptors) revealed selective delivery and destruction of PC-3 cells using targeted Pt-peptide conjugates 7 and 8 significantly more than untargeted carboplatin. Platinum uptake on PC-3 cells was 12-fold more for conjugate 7 and 3-fold more for conjugate 8 compared to that of the untargeted carboplatin, indicating selective activation of the CD13 receptors and delivery of the conjugates to CD13 positive cells. Further analysis on effects of conjugates 7 and 8 on PC-3 cells using caspase-3/7, fluorescence microscopy, and DNA fragmentation confirmed that the cells were dying by apoptosis.
Figures







Similar articles
-
A doxorubicin-CNGRC-peptide conjugate with prodrug properties.Biochem Pharmacol. 2002 Mar 1;63(5):897-908. doi: 10.1016/s0006-2952(01)00928-5. Biochem Pharmacol. 2002. PMID: 11911842
-
The CNGRC-GG-D(KLAKLAK)2 peptide induces a caspase-independent, Ca2+-dependent death in human leukemic myeloid cells by targeting surface aminopeptidase N/CD13.Oncotarget. 2016 Apr 12;7(15):19445-67. doi: 10.18632/oncotarget.6523. Oncotarget. 2016. PMID: 26655501 Free PMC article.
-
Conjugated platinum(IV)-peptide complexes for targeting angiogenic tumor vasculature.Bioconjug Chem. 2008 Jan;19(1):39-49. doi: 10.1021/bc070031k. Epub 2007 Sep 11. Bioconjug Chem. 2008. PMID: 17845003 Free PMC article.
-
Nuclear localization signal-targeted poly(ethylene glycol) conjugates as potential carriers and nuclear localizing agents for carboplatin analogues.Bioconjug Chem. 2004 Jul-Aug;15(4):814-23. doi: 10.1021/bc0499331. Bioconjug Chem. 2004. PMID: 15264869
-
Synthesis and in vitro evaluation of cyclic NGR peptide targeted thermally sensitive liposome.J Control Release. 2010 Apr 19;143(2):265-73. doi: 10.1016/j.jconrel.2009.12.031. Epub 2010 Jan 11. J Control Release. 2010. PMID: 20067811 Free PMC article.
Cited by
-
In Vivo Tumor Growth Inhibition and Antiangiogenic Effect of Cyclic NGR Peptide-Daunorubicin Conjugates Developed for Targeted Drug Delivery.Pathol Oncol Res. 2020 Jul;26(3):1879-1892. doi: 10.1007/s12253-019-00773-3. Epub 2019 Dec 9. Pathol Oncol Res. 2020. PMID: 31820302 Free PMC article.
-
Biodistribution and SPECT imaging study of (99m)Tc labeling NGR peptide in nude mice bearing human HepG2 hepatoma.Biomed Res Int. 2014;2014:618096. doi: 10.1155/2014/618096. Epub 2014 May 19. Biomed Res Int. 2014. PMID: 24977153 Free PMC article.
-
Engineering and Validation of a Peptide-Stabilized Poly(lactic-co-glycolic) Acid Nanoparticle for Targeted Delivery of a Vascular Disruptive Agent in Cancer Therapy.Bioconjug Chem. 2022 Dec 21;33(12):2348-2360. doi: 10.1021/acs.bioconjchem.2c00418. Epub 2022 Nov 11. Bioconjug Chem. 2022. PMID: 36367382 Free PMC article.
-
Synthesis and evaluation of novel Tc-99m labeled probestin conjugates for imaging APN/CD13 expression in vivo.Bioconjug Chem. 2012 Jan 18;23(1):115-24. doi: 10.1021/bc200546b. Epub 2011 Dec 20. Bioconjug Chem. 2012. PMID: 22148582 Free PMC article.
-
Lentivirus vector-mediated gene transduction of CNGRC peptide in rat adipose stem cells.Mol Med Rep. 2015 Apr;11(4):2555-61. doi: 10.3892/mmr.2014.3043. Epub 2014 Dec 3. Mol Med Rep. 2015. PMID: 25482186 Free PMC article.
References
-
- Wang D, Lippard SJ. Cellular processing of platinum anticancer drugs. Nat. Rev. Drug Discovery. 2005;4:307–320. - PubMed
-
- Reedijk J. Improved understanding in platinum antitumor chemistry. Chem. Commun. (Cambridge) 1996:801–6.
-
- Shipp M, Look T. Hematopoietic Differentiation Antigens That Are Membrane-Associated Enzymes: Cutting Is the Key! Blood. 1993;82:1052–1070. - PubMed
-
- Jamieson E, Lippard S. Structure, Recognition, and Processing of Cisplatin-DNA Adducts. Chem. Rev. (Washington, DC, U. S.) 1999;99:2467–2498. - PubMed
-
- Hamelers IHL, Van Loenen E, Staffhorst RWHM, De Kruijff B, De Kroon AIPM. Carboplatin nanocapsules: a highly cytotoxic, phospholipid-based formulation of carboplatin. Mol. Cancer Ther. 2006;5:2007–2012. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

