Cannibalism enhances biofilm development in Bacillus subtilis
- PMID: 19775247
- PMCID: PMC2983100
- DOI: 10.1111/j.1365-2958.2009.06882.x
Cannibalism enhances biofilm development in Bacillus subtilis
Abstract
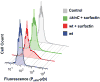
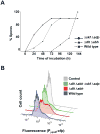
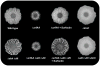
Cannibalism is a mechanism to delay sporulation in Bacillus subtilis. Cannibal cells express the skf and sdp toxin systems to lyse a fraction of their sensitive siblings. The lysed cells release nutrients that serve to feed the community, effectively delaying spore formation. Here we provide evidence that the subpopulation of cells that differentiates into cannibals is the same subpopulation that produces the extracellular matrix that holds cells together in biofilms. Cannibalism and matrix formation are both triggered in response to the signalling molecule surfactin. Nutrients released by the cannibalized cells are preferentially used by matrix-producing cells, as they are the only cells expressing resistance to the Skf and Sdp toxins. As a result this subpopulation increases in number and matrix production is enhanced when cannibalism toxins are produced. The cannibal/matrix-producing subpopulation is also generated in response to antimicrobials produced by other microorganisms and may thus constitute a defense mechanism to protect B. subtilis from the action of antibiotics in natural settings.
Figures




Similar articles
-
Cannibalism stress response in Bacillus subtilis.Microbiology (Reading). 2016 Jan;162(1):164-176. doi: 10.1099/mic.0.000176. Epub 2015 Sep 11. Microbiology (Reading). 2016. PMID: 26364265
-
Control of cell fate by the formation of an architecturally complex bacterial community.Genes Dev. 2008 Apr 1;22(7):945-53. doi: 10.1101/gad.1645008. Genes Dev. 2008. PMID: 18381896 Free PMC article.
-
Cannibalism: a social behavior in sporulating Bacillus subtilis.FEMS Microbiol Rev. 2011 May;35(3):415-24. doi: 10.1111/j.1574-6976.2010.00253.x. Epub 2010 Oct 19. FEMS Microbiol Rev. 2011. PMID: 20955377 Review.
-
Biofilm-associated toxin and extracellular protease cooperatively suppress competitors in Bacillus subtilis biofilms.PLoS Genet. 2019 Oct 17;15(10):e1008232. doi: 10.1371/journal.pgen.1008232. eCollection 2019 Oct. PLoS Genet. 2019. PMID: 31622331 Free PMC article.
-
Biofilm formation by Bacillus subtilis: new insights into regulatory strategies and assembly mechanisms.Mol Microbiol. 2014 Aug;93(4):587-98. doi: 10.1111/mmi.12697. Epub 2014 Jul 18. Mol Microbiol. 2014. PMID: 24988880 Free PMC article. Review.
Cited by
-
Biofilm inhibitors that target amyloid proteins.Chem Biol. 2013 Jan 24;20(1):102-10. doi: 10.1016/j.chembiol.2012.10.021. Chem Biol. 2013. PMID: 23352144 Free PMC article.
-
Pulsed feedback defers cellular differentiation.PLoS Biol. 2012 Jan;10(1):e1001252. doi: 10.1371/journal.pbio.1001252. Epub 2012 Jan 31. PLoS Biol. 2012. PMID: 22303282 Free PMC article.
-
Bacteriophage-mediated lysis supports robust growth of amino acid auxotrophs.bioRxiv [Preprint]. 2023 Mar 1:2023.02.28.530524. doi: 10.1101/2023.02.28.530524. bioRxiv. 2023. Update in: ISME J. 2023 Oct;17(10):1785-1788. doi: 10.1038/s41396-023-01452-7. PMID: 36909566 Free PMC article. Updated. Preprint.
-
Role of Glutamate Synthase in Biofilm Formation by Bacillus subtilis.J Bacteriol. 2020 Jun 25;202(14):e00120-20. doi: 10.1128/JB.00120-20. Print 2020 Jun 25. J Bacteriol. 2020. PMID: 32393519 Free PMC article.
-
Density of founder cells affects spatial pattern formation and cooperation in Bacillus subtilis biofilms.ISME J. 2014 Oct;8(10):2069-79. doi: 10.1038/ismej.2014.52. Epub 2014 Apr 3. ISME J. 2014. PMID: 24694715 Free PMC article.
References
-
- Branda SS, Chu F, Kearns DB, Losick R, Kolter R. A major protein component of the Bacillus subtilis biofilm matrix. Mol Microbiol. 2006;59:1229–1238. - PubMed
-
- Buchman GW, Banerjee S, Hansen JN. Structure, expression, and evolution of a gene encoding the precursor of nisin, a small protein antibiotic. J Biol Chem. 1988;263:16260–16266. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

