A secretory phospholipase A2-mediated neuroprotection and anti-apoptosis
- PMID: 19775433
- PMCID: PMC2758888
- DOI: 10.1186/1471-2202-10-120
A secretory phospholipase A2-mediated neuroprotection and anti-apoptosis
Abstract
Background: Phospholipase A2 liberates free fatty acids and lysophospholipids upon hydrolysis of phospholipids and these products are often associated with detrimental effects such as inflammation and cerebral ischemia. The neuroprotective effect of neutral phospholipase from snake venom has been investigated.
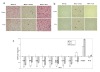
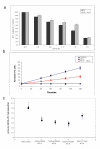
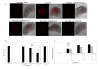
Results: A neutral anticoagulant secretory phospholipase A2 (nPLA) from the venom of Naja sputatrix (Malayan spitting cobra) has been found to reduce infarct volume in rats subjected to focal transient cerebral ischemia and to alleviate the neuronal damage in organotypic hippocampal slices subjected to oxygen-glucose deprivation (OGD). Real-time PCR based gene expression analysis showed that anti-apoptotic and pro-survival genes have been up-regulated in both in vivo and in vitro models. Staurosporine or OGD mediated apoptotic cell death in astrocytoma cells has also been found to be reduced by nPLA with a corresponding reduction in caspase 3 activity.
Conclusion: We have found that a secretory phospholipase (nPLA) purified from snake venom could reduce infarct volume in rodent stroke model. nPLA, has also been found to reduce neuronal cell death, apoptosis and promote cell survival in vitro ischemic conditions. In all conditions, the protective effects could be seen at sub-lethal concentrations of the protein.
Figures
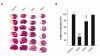
References
-
- Dennis EA. Diversity of group types, regulation, and function of phospholipase A2. J Biol Chem. 1994;269:13057–60. - PubMed
-
- Cummings BS, McHowat J, Schnellmann RG. Phospholipase A(2)s in cell injury and death. J Pharmacol Exp Ther. 2000;294:793–9. - PubMed
-
- Winstead MV, Balsinde J, Dennis EA. Calcium-independent phospholipase A(2): structure and function. Biochim Biophys Acta. 2000;1488:28–39. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

