Parkinson's disease brain mitochondria have impaired respirasome assembly, age-related increases in distribution of oxidative damage to mtDNA and no differences in heteroplasmic mtDNA mutation abundance
- PMID: 19775436
- PMCID: PMC2761382
- DOI: 10.1186/1750-1326-4-37
Parkinson's disease brain mitochondria have impaired respirasome assembly, age-related increases in distribution of oxidative damage to mtDNA and no differences in heteroplasmic mtDNA mutation abundance
Abstract
Background: Sporadic Parkinson's disease (sPD) is a nervous system-wide disease that presents with a bradykinetic movement disorder and is frequently complicated by depression and cognitive impairment. sPD likely has multiple interacting causes that include increased oxidative stress damage to mitochondrial components and reduced mitochondrial bioenergetic capacity. We analyzed mitochondria from postmortem sPD and CTL brains for evidence of oxidative damage to mitochondrial DNA (mtDNA), heteroplasmic mtDNA point mutations and levels of electron transport chain proteins. We sought to determine if sPD brains possess any mtDNA genotype-respiratory phenotype relationships.
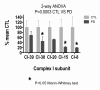
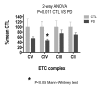
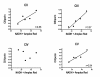
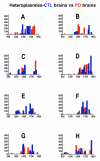
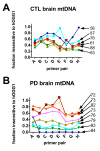
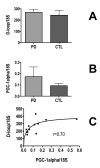
Results: Treatment of sPD brain mtDNA with the mitochondrial base-excision repair enzyme 8-oxyguanosine glycosylase-1 (hOGG1) inhibited, in an age-dependent manner, qPCR amplification of overlapping ~2 kbase products; amplification of CTL brain mtDNA showed moderate sensitivity to hOGG1 not dependent on donor age. hOGG1 mRNA expression was not different between sPD and CTL brains. Heteroplasmy analysis of brain mtDNA using Surveyor nuclease(R) showed asymmetric distributions and levels of heteroplasmic mutations across mtDNA but no patterns that statistically distinguished sPD from CTL. sPD brain mitochondria displayed reductions of nine respirasome proteins (respiratory complexes I-V). Reduced levels of sPD brain mitochondrial complex II, III and V, but not complex I or IV proteins, correlated closely with rates of NADH-driven electron flow. mtDNA levels and PGC-1alpha expression did not differ between sPD and CTL brains.
Conclusion: PD brain mitochondria have reduced mitochondrial respiratory protein levels in complexes I-V, implying a generalized defect in respirasome assembly. These deficiencies do not appear to arise from altered point mutational burden in mtDNA or reduction of nuclear signaling for mitochondrial biogenesis, implying downstream etiologies. The origin of age-related increases in distribution of oxidative mtDNA damage in sPD but not CTL brains is not clear, tracks with but does not determine the sPD phenotype, and may indicate a unique consequence of aging present in sPD that could contribute to mtDNA deletion generation in addition to mtDNA replication, transcription and sequencing errors. sPD frontal cortex experiences a generalized bioenergetic deficiency above and beyond aging that could contribute to mood disorders and cognitive impairments.
Figures
References
-
- Bertrand E, Lechowicz W, Szpak GM, Lewandowska E, Dymecki J, Wierzba-Bobrowicz T. Limbic neuropathology in idiopathic Parkinson's disease with concomitant dementia. Folia Neuropathol. 2004;42:141–150. - PubMed
-
- Braak E, Sandmann-Keil D, Rub U, Gai WP, de Vos RA, Steur EN, Arai K, Braak H. alpha-synuclein immunopositive Parkinson's disease-related inclusion bodies in lower brain stem nuclei. Acta Neuropathol (Berl) 2001;101:195–201. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

