The Irish DAFNE study protocol: a cluster randomised trial of group versus individual follow-up after structured education for type 1 diabetes
- PMID: 19775465
- PMCID: PMC2761911
- DOI: 10.1186/1745-6215-10-88
The Irish DAFNE study protocol: a cluster randomised trial of group versus individual follow-up after structured education for type 1 diabetes
Abstract
Background: Structured education programmes for individuals with Type 1 diabetes have become a recognised means of delivering the knowledge and skills necessary for optimal self-management of the condition. The Dose Adjustment for Normal Eating (DAFNE) programme has been shown to improve biomedical (HbA(1c) and rates of severe hypoglycaemia) and psychosocial outcomes for up to 12 months following course delivery. The optimal way to support DAFNE graduates and maintain the benefits of the programme has not been established. We aimed to compare 2 different methods of follow-up of DAFNE graduates in a pragmatic clinical trial delivered in busy diabetes clinics on the island of Ireland.
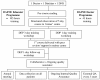
Methods: Six participating centres were cluster randomised to deliver either group follow-up or a return to traditional one-to-one clinic visits. In the intervention arm group follow-up was delivered at 6 and 12 months post DAFNE training according to a curriculum developed for the study. In the control arm patients were seen individually in diabetes clinics as part of routine care. Study outcomes included HbA(1c) levels, self-reported rates of severe hypoglycaemia, body weight and measures of diabetes wellbeing and quality of life. These were measured at 6, 12 and 18 months after recruitment. Generalisability (external validity) was maximised by recruiting study participants from existing DAFNE waiting lists in each centre, by using broad inclusion criteria (including HbA(1c) values less than 13 percent with no lower limit) and by using existing clinic staff to deliver the training and follow-up. Internal validity and treatment fidelity were maximised by quality assuring the training of all DAFNE educators, by external peer review of the group follow-up sessions and by striving for full attendance at follow-up visits. Assays of HbA(1c) were undertaken in a central laboratory.
Discussion: This pragmatic clinical trial evaluating group follow-up after a structured education programme has been designed to have broad generalisability. The results should inform how best to manage the well educated patient with Type 1 diabetes in the real world of clinical practice
Trial registration: Current Controlled Trials ISRCTN79759174.
Figures
References
-
- National Diabetes Audit Executive Summary Key findings about the quality of care for people with diabetes in England and Wales. Report for the audit period 2007-2008. The Information Centre for Health and Social Care. 2008 doi: 10.1136/adc.2004.061150. - DOI
-
- Therapeutic Patient Education. Continuing Education Programmes for Health Care Providers in the Field of Prevention of Chronic Diseases. Report of a World Health Organisation Working Group. 1998 doi: 10.1007/s00125-005-1905-1. - DOI
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous