Control of cell growth by the SCF and APC/C ubiquitin ligases
- PMID: 19775879
- PMCID: PMC2805079
- DOI: 10.1016/j.ceb.2009.08.004
Control of cell growth by the SCF and APC/C ubiquitin ligases
Abstract
The ubiquitin-proteasome system plays key roles in the control of cell growth. The cell cycle, in particular, is highly regulated by the functions of the SCF and APC/C ubiquitin ligases, and perturbation of their function can result in tumorigenesis. Although the SCF and APC/C complexes are well established in growth control pathways, many aspects of their function remain unknown. Recent studies have shed light on the mechanism of SCF-mediated ubiquitination and new functions for the SCF complex and APC/C. Our expanding understanding of the roles of the SCF and APC/C complexes highlight the potential for targeted molecular therapies.
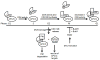
Figures


References
-
- Pickart CM, Fushman D. Polyubiquitin chains: polymeric protein signals. Curr Opin Chem Biol. 2004;8:610–616. - PubMed
-
- Hershko A, Ciechanover A. The ubiquitin system. Annu Rev Biochem. 1998;67:425–479. - PubMed
-
- Schwartz AL, Ciechanover A. The ubiquitin-proteasome pathway and pathogenesis of human diseases. Annu Rev Med. 1999;50:57–74. - PubMed
-
- Weissman AM. Themes and variations on ubiquitylation. Nat Rev Mol Cell Biol. 2001;2:169–178. - PubMed
-
- Cardozo T, Pagano M. The SCF ubiquitin ligase: insights into a molecular machine. Nat Rev Mol Cell Biol. 2004;5:739–751. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

