Cholesterol induces renal vasoconstriction and anti-natriuresis by inhibiting nitric oxide production in anesthetized rats
- PMID: 19776170
- PMCID: PMC2801331
- DOI: 10.1152/ajprenal.90743.2008
Cholesterol induces renal vasoconstriction and anti-natriuresis by inhibiting nitric oxide production in anesthetized rats
Abstract
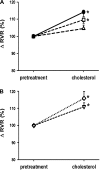
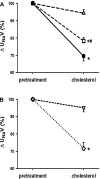
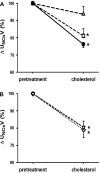
Although hypercholesterolemia is implicated in the pathophysiology of many renal disorders as well as hypertension, its direct actions in the kidney are not yet clearly understood. In the present study, we evaluated renal responses to administration of cholesterol (8 microg x min(-1).100 g body wt(-1); bound by polyethylene glycol) into the renal artery of anesthetized male Sprague-Dawley rats. Total renal blood flow (RBF) was measured by a Transonic flow probe, and glomerular filtration rate (GFR) was determined by Inulin clearance. In control rats (n = 8), cholesterol induced reductions of 10 +/- 2% in RBF [baseline (b) 7.6 +/- 0.3 microg x min(-1).100 g(-1)], 17 +/- 3% in urine flow (b, 10.6 +/- 0.9 microg x min(-1).100 g(-1)), 29 +/- 3% in sodium excretion (b, 0.96 +/- 0.05 mumol.min(-1).100 g(-1)) and 24 +/- 2% in nitrite/nitrate excretion (b, 0.22 +/- 0.01 nmol.min(-1).100 g(-1)) without an appreciable change in GFR (b, 0.87 +/- 0.03 ml.min(-1).100 g(-1)). These renal vasoconstrictor and anti-natriuretic responses to cholesterol were absent in rats pretreated with nitric oxide (NO) synthase inhibitor, nitro-l-arginine methylester (0.5 microg x min(-1).100 g(-1); n = 6). In rats pretreated with superoxide (O(2)(-)) scavenger tempol (50 microg x min(-1).100 g(-1); n = 6), the cholesterol-induced renal responses remained mostly unchanged, although there was a slight attenuation in anti-natriuretic response. This anti-natriuretic response to cholesterol was abolished in furosemide-pretreated rats (0.3 microg x min(-1).100 g(-1); n = 6) but remained unchanged in amiloride-pretreated rats (0.2 microg x min(-1).100 g(-1); n = 5), indicating that Na(+)/K(+)/2Cl(-) cotransport is the dominant mediator of this effect. These data demonstrate that cholesterol-induced acute renal vasoconstrictor and antinatriuretic responses are mediated by a decrease in NO production. These data also indicate that tubular effect of cholesterol on sodium reabsorption is mediated by the furosemide sensitive Na(+)/K(+)/2Cl(-) cotransporter.
Figures





References
-
- Attia DM, Feron O, Goldschmeding R, Radermakers LH, Vaziri ND, Boer P, Balligand JL, Koomans HA, Joles JA. Hypercholesterolemia in rats induces podocyte stress and decreases renal cortical nitric oxide synthesis via an angiotensin II type 1 receptor-sensitive mechanism. J Am Soc Nephrol 15: 949–957, 2004 - PubMed
-
- Attia DM, Ni ZN, Boer P, Attia MA, Goldschmeding R, Koomans HA, Vaziri ND, Joles JA. Proteinuria is preceded by decreased nitric oxide synthesis and prevented by a NO donor in cholesterol-fed rats. Kidney Int 61: 1776–1787, 2002 - PubMed
-
- Balut C, Steels P, Radu M, Ameloot M, Driessche WV, Jans D. Membrane cholesterol extraction decreases Na+ transport in A6 renal epithelia. Am J Physiol Cell Physiol 290: C87–C94, 2006 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

