Motoneuron transplantation rescues the phenotype of SMARD1 (spinal muscular atrophy with respiratory distress type 1)
- PMID: 19776263
- PMCID: PMC6666655
- DOI: 10.1523/JNEUROSCI.2734-09.2009
Motoneuron transplantation rescues the phenotype of SMARD1 (spinal muscular atrophy with respiratory distress type 1)
Abstract
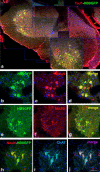
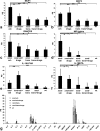
Spinal muscular atrophy with respiratory distress type 1 (SMARD1) is a fatal form of infantile motoneuron disease. There is currently no effective treatment, although motor neuron replacement is a possible therapeutic strategy. We transplanted purified motor neurons into the spinal cord of nmd mice, an animal model of SMARD1. We also administered pharmacological treatment targeting the induction of axonal growth toward skeletal muscle target. At the end stage of the disease, donor-derived motor neurons were detected in the nmd anterior horns, extended axons into the ventral roots, and formed new neuromuscular junctions. These data correlated with improved neuromuscular function and increased life spans. The neuroprotective effect was associated with a reduction in proinflammatory molecules in treated spinal cords. This is the first report that functional restoration of motor units with transplanted motoneurons is feasible in an animal model of a human motoneuron disease, opening up new possibilities for therapeutic intervention.
Figures




Similar articles
-
Transplanted ALDHhiSSClo neural stem cells generate motor neurons and delay disease progression of nmd mice, an animal model of SMARD1.Hum Mol Genet. 2006 Jan 15;15(2):167-87. doi: 10.1093/hmg/ddi446. Epub 2005 Dec 8. Hum Mol Genet. 2006. PMID: 16339214
-
Fast motor axon loss in SMARD1 does not correspond to morphological and functional alterations of the NMJ.Neurobiol Dis. 2013 Jun;54:169-82. doi: 10.1016/j.nbd.2012.12.010. Epub 2013 Jan 4. Neurobiol Dis. 2013. PMID: 23295857
-
Characterization of Ighmbp2 in motor neurons and implications for the pathomechanism in a mouse model of human spinal muscular atrophy with respiratory distress type 1 (SMARD1).Hum Mol Genet. 2004 Sep 15;13(18):2031-42. doi: 10.1093/hmg/ddh222. Epub 2004 Jul 21. Hum Mol Genet. 2004. PMID: 15269181
-
The role of embryonic motoneuron transplants to restore the lost motor function of the injured spinal cord.Ann Anat. 2011 Jul;193(4):362-70. doi: 10.1016/j.aanat.2011.04.001. Epub 2011 Apr 30. Ann Anat. 2011. PMID: 21600746 Review.
-
Stem cell-derived neurotrophic support for the neuromuscular junction in spinal muscular atrophy.Expert Opin Biol Ther. 2010 Nov;10(11):1587-94. doi: 10.1517/14712598.2010.529895. Expert Opin Biol Ther. 2010. PMID: 20955113 Review.
Cited by
-
Generating spinal motor neuron diversity: a long quest for neuronal identity.Cell Mol Life Sci. 2014 Mar;71(5):813-29. doi: 10.1007/s00018-013-1398-x. Epub 2013 Jun 14. Cell Mol Life Sci. 2014. PMID: 23765105 Free PMC article. Review.
-
Human pluripotent stem cells: applications and challenges in neurological diseases.Front Physiol. 2012 Jul 20;3:267. doi: 10.3389/fphys.2012.00267. eCollection 2012. Front Physiol. 2012. PMID: 22934023 Free PMC article.
-
Spinal muscular atrophy: mechanisms and therapeutic strategies.Hum Mol Genet. 2010 Apr 15;19(R1):R111-8. doi: 10.1093/hmg/ddq147. Epub 2010 Apr 13. Hum Mol Genet. 2010. PMID: 20392710 Free PMC article. Review.
-
Gene therapy rescues disease phenotype in a spinal muscular atrophy with respiratory distress type 1 (SMARD1) mouse model.Sci Adv. 2015 Mar 13;1(2):e1500078. doi: 10.1126/sciadv.1500078. eCollection 2015 Mar. Sci Adv. 2015. PMID: 26601156 Free PMC article.
-
Stem cell transplantation for motor neuron disease: current approaches and future perspectives.Neurotherapeutics. 2011 Oct;8(4):591-606. doi: 10.1007/s13311-011-0068-7. Neurotherapeutics. 2011. PMID: 21904789 Free PMC article. Review.
References
-
- Arce V, Garces A, de Bovis B, Filippi P, Henderson C, Pettmann B, deLapeyrière O. Cardiotrophin-1 requires LIFRbeta to promote survival of mouse motoneurons purified by a novel technique. J Neurosci Res. 1999;55:119–126. - PubMed
-
- Baron P, Bussini S, Cardin V, Corbo M, Conti G, Galimberti D, Scarpini E, Bresolin N, Wharton SB, Shaw PJ, Silani V. Production of monocyte chemoattractant protein-1 in amyotrophic lateral sclerosis. Muscle Nerve. 2005;32:541–544. - PubMed
-
- Corti S, Locatelli F, Papadimitriou D, Donadoni C, Del Bo R, Fortunato F, Strazzer S, Salani S, Bresolin N, Comi GP. Multipotentiality, homing properties, and pyramidal neurogenesis of CNS-derived LeX(ssea-1)+/CXCR4+ stem cells. FASEB J. 2005;19:1860–1862. - PubMed
-
- Corti S, Locatelli F, Papadimitriou D, Donadoni C, Del Bo R, Crimi M, Bordoni A, Fortunato F, Strazzer S, Menozzi G, Salani S, Bresolin N, Comi GP. Transplanted ALDHhiSSClo neural stem cells generate motor neurons and delay disease progression of nmd mice, an animal model of SMARD1. Hum Mol Genet. 2006;15:167–187. - PubMed
-
- Corti S, Locatelli F, Papadimitriou D, Del Bo R, Nizzardo M, Nardini M, Donadoni C, Salani S, Fortunato F, Strazzer S, Bresolin N, Comi GP. Neural stem cells LewisX+ CXCR4+ modify disease progression in an amyotrophic lateral sclerosis model. Brain. 2007;130:1289–1305. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
