Encoding and decoding bursts by NMDA spikes in basal dendrites of layer 5 pyramidal neurons
- PMID: 19776275
- PMCID: PMC3850222
- DOI: 10.1523/JNEUROSCI.5250-08.2009
Encoding and decoding bursts by NMDA spikes in basal dendrites of layer 5 pyramidal neurons
Abstract
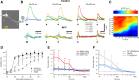
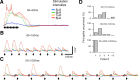
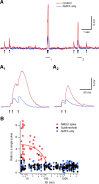
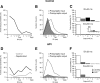
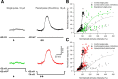
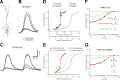
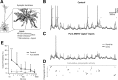
Bursts of action potentials are important information-bearing signals in the brain, although the neuronal specializations underlying burst generation and detection are only partially understood. In apical dendrites of neocortical pyramidal neurons, calcium spikes are known to contribute to burst generation, but a comparable understanding of basal dendritic mechanisms is lacking. Here we show that NMDA spikes in basal dendrites mediate both detection and generation of bursts through a postsynaptic mechanism. High-frequency inputs to basal dendrites markedly facilitated NMDA spike initiation compared with low-frequency activation or single inputs. Unlike conventional temporal summation effects based on voltage, however, NMDA spike facilitation depended mainly on residual glutamate bound to NMDA receptors from previous activations. Once triggered by an input burst, we found that NMDA spikes in turn reliably trigger output bursts under in vivo-like stimulus conditions. Through their unique biophysical properties, NMDA spikes are thus ideally suited to promote the propagation of bursts through the cortical network.
Figures



Similar articles
-
NMDA spikes in basal dendrites of cortical pyramidal neurons.Nature. 2000 Mar 16;404(6775):285-9. doi: 10.1038/35005094. Nature. 2000. PMID: 10749211
-
Glutamate spillover promotes the generation of NMDA spikes.J Neurosci. 2011 Nov 9;31(45):16435-46. doi: 10.1523/JNEUROSCI.2777-11.2011. J Neurosci. 2011. PMID: 22072693 Free PMC article.
-
Calcium spikes in basal dendrites of layer 5 pyramidal neurons during action potential bursts.J Neurosci. 2006 Jul 12;26(28):7424-32. doi: 10.1523/JNEUROSCI.3062-05.2006. J Neurosci. 2006. PMID: 16837590 Free PMC article.
-
The decade of the dendritic NMDA spike.J Neurosci Res. 2010 Nov 1;88(14):2991-3001. doi: 10.1002/jnr.22444. J Neurosci Res. 2010. PMID: 20544831 Free PMC article. Review.
-
Active properties of neocortical pyramidal neuron dendrites.Annu Rev Neurosci. 2013 Jul 8;36:1-24. doi: 10.1146/annurev-neuro-062111-150343. Annu Rev Neurosci. 2013. PMID: 23841837 Review.
Cited by
-
Dopaminergic control of motivation and reinforcement learning: a closed-circuit account for reward-oriented behavior.J Neurosci. 2013 May 15;33(20):8866-90. doi: 10.1523/JNEUROSCI.4614-12.2013. J Neurosci. 2013. PMID: 23678129 Free PMC article.
-
Autism candidate gene DIP2A regulates spine morphogenesis via acetylation of cortactin.PLoS Biol. 2019 Oct 10;17(10):e3000461. doi: 10.1371/journal.pbio.3000461. eCollection 2019 Oct. PLoS Biol. 2019. PMID: 31600191 Free PMC article.
-
Input clustering and the microscale structure of local circuits.Front Neural Circuits. 2014 Sep 12;8:112. doi: 10.3389/fncir.2014.00112. eCollection 2014. Front Neural Circuits. 2014. PMID: 25309336 Free PMC article. Review.
-
Altered integration of excitatory inputs onto the basal dendrites of layer 5 pyramidal neurons in a mouse model of Fragile X syndrome.Proc Natl Acad Sci U S A. 2023 Jan 10;120(2):e2208963120. doi: 10.1073/pnas.2208963120. Epub 2023 Jan 3. Proc Natl Acad Sci U S A. 2023. PMID: 36595706 Free PMC article.
-
Glutamate-bound NMDARs arising from in vivo-like network activity extend spatio-temporal integration in a L5 cortical pyramidal cell model.PLoS Comput Biol. 2014 Apr 24;10(4):e1003590. doi: 10.1371/journal.pcbi.1003590. eCollection 2014 Apr. PLoS Comput Biol. 2014. PMID: 24763087 Free PMC article.
References
-
- Abbott LF, Regehr WG. Synaptic computation. Nature. 2004;431:796–803. - PubMed
-
- Akopian G, Walsh JP. Corticostriatal paired-pulse potentiation produced by voltage-dependent activation of NMDA receptors and L-type Ca2+ channels. J Neurophysiol. 2002;87:157–165. - PubMed
-
- Cattaneo A, Maffei L, Morrone C. Two firing patterns in the discharge of complex cells encoding different attributes of the visual stimulus. Exp Brain Res. 1981;43:115–118. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
