CD14 and toll-like receptors 2 and 4 are required for fibrillar A{beta}-stimulated microglial activation
- PMID: 19776284
- PMCID: PMC2778845
- DOI: 10.1523/JNEUROSCI.3158-09.2009
CD14 and toll-like receptors 2 and 4 are required for fibrillar A{beta}-stimulated microglial activation
Abstract
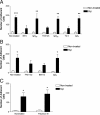
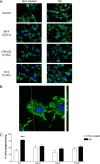
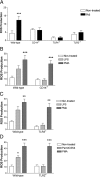
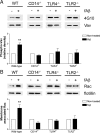
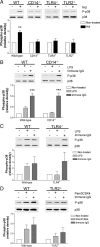
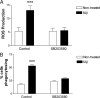
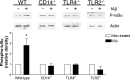
Microglia are the brain's tissue macrophages and are found in an activated state surrounding beta-amyloid plaques in the Alzheimer's disease brain. Microglia interact with fibrillar beta-amyloid (fAbeta) through an ensemble of surface receptors composed of the alpha(6)beta(1) integrin, CD36, CD47, and the class A scavenger receptor. These receptors act in concert to initiate intracellular signaling cascades and phenotypic activation of these cells. However, it is unclear how engagement of this receptor complex is linked to the induction of an activated microglial phenotype. We report that the response of microglial cells to fibrillar forms of Abeta requires the participation of Toll-like receptors (TLRs) and the coreceptor CD14. The response of microglia to fAbeta is reliant upon CD14, which act together with TLR4 and TLR2 to bind fAbeta and to activate intracellular signaling. We find that cells lacking these receptors could not initiate a Src-Vav-Rac signaling cascade leading to reactive oxygen species production and phagocytosis. The fAbeta-mediated activation of p38 MAPK also required CD14, TLR4, and TLR2. Inhibition of p38 abrogated fAbeta-induced reactive oxygen species production and attenuated the induction of phagocytosis. Microglia lacking CD14, TLR4, and TLR2 showed no induction of phosphorylated IkappaBalpha following fAbeta. These data indicate these innate immune receptors function as members of the microglial fAbeta receptor complex and identify the signaling mechanisms whereby they contribute to microglial activation.
Figures

References
-
- Aderem A. How to eat something bigger than your head. Cell. 2002;110:5–8. - PubMed
-
- Aderem A, Underhill DM. Mechanisms of phagocytosis in macrophages. Annu Rev Immunol. 1999;17:593–623. - PubMed
-
- Ago T, Nunoi H, Ito T, Sumimoto H. Mechanism for phosphorylation-induced activation of the phagocyte NADPH oxidase protein p47(phox). Triple replacement of serines 303, 304, and 328 with aspartates disrupts the SH3 domain-mediated intramolecular interaction in p47(phox), thereby activating the oxidase. J Biol Chem. 1999;274:33644–33653. - PubMed
-
- Akira S. Toll-like receptors and innate immunity. Adv Immunol. 2001;78:1–56. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
