A chimera analysis of prestin knock-out mice
- PMID: 19776286
- PMCID: PMC2801582
- DOI: 10.1523/JNEUROSCI.1651-09.2009
A chimera analysis of prestin knock-out mice
Abstract
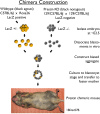
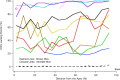
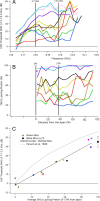
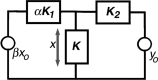
A chimera is a genetic composite containing a unique mix of cells derived from more than one zygote. This mouse model allows one to learn how cells of contrasting genotype functionally interact in vivo. Here, we investigate the effect that different proportions of prestin-containing outer hair cells (OHC) have on cochlear amplification. To address this issue, we developed a prestin chimeric mouse in which both ROSA26 wild-type (WT) and prestin knock-out (KO) genotypes are present in a single cochlea. The WT ROSA26 mice express a cell marker, allowing one to identify cells originating from the WT genome. Examination of cochlear tissue indicated that prestin chimeric mice demonstrate a mosaic in which mutant and normal OHCs interleave along the cochlear partition, similar to all other chimeric mouse models. The anatomical distribution of prestin-containing OHCs was compared with physiological data including thresholds and tuning curves for the compound action potential (CAP) recorded in anesthetized mice. Analysis of these measures did not reveal mixed phenotypes in which the distribution of prestin-containing OHCs impacted sensitivity and frequency selectivity to different degrees. However, by reducing the number of prestin-containing OHCs, phenotypes intermediate between WT and KO response patterns were obtained. Accordingly, we demonstrate a proportional reduction in sensitivity and in the tip length of CAP tuning curves as the number of OHCs derived from the KO genome increases; i.e., genotype ratio and phenotype are closely related.
Figures






References
-
- Bohne BA, Harding GW. Processing and analyzing the mouse temporal bone to identify gross, cellular and subcellular pathology. Hear Res. 1997;109:34–45. - PubMed
-
- Brown SD, Hardisty-Hughes RE, Mburu P. Quiet as a mouse: dissecting the molecular and genetic basis of hearing. Nat Rev Genet. 2008;9:277–290. - PubMed
-
- Cheatham MA, Zheng J, Huynh KH, Du GG, Edge RM, Anderson CT, Zuo J, Ryan AF, Dallos P. Evaluation of an independent prestin mouse model derived from the 129S1 strain. Audiol Neurootol. 2007;12:378–390. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
