Combined immunodeficiency associated with DOCK8 mutations
- PMID: 19776401
- PMCID: PMC2965730
- DOI: 10.1056/NEJMoa0905506
Combined immunodeficiency associated with DOCK8 mutations
Abstract
Background: Recurrent sinopulmonary and cutaneous viral infections with elevated serum levels of IgE are features of some variants of combined immunodeficiency. The genetic causes of these variants are unknown.
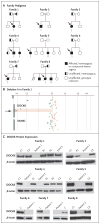
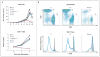
Methods: We collected longitudinal clinical data on 11 patients from eight families who had recurrent sinopulmonary and cutaneous viral infections. We performed comparative genomic hybridization arrays and targeted gene sequencing. Variants with predicted loss-of-expression mutations were confirmed by means of a quantitative reverse-transcriptase-polymerase-chain-reaction assay and immunoblotting. We evaluated the number and function of lymphocytes with the use of in vitro assays and flow cytometry.
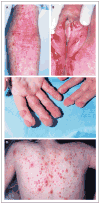
Results: Patients had recurrent otitis media, sinusitis, and pneumonias; recurrent Staphylococcus aureus skin infections with otitis externa; recurrent, severe herpes simplex virus or herpes zoster infections; extensive and persistent infections with molluscum contagiosum; and human papillomavirus infections. Most patients had severe atopy with anaphylaxis; several had squamous-cell carcinomas, and one had T-cell lymphoma-leukemia. Elevated serum IgE levels, hypereosinophilia, low numbers of T cells and B cells, low serum IgM levels, and variable IgG antibody responses were common. Expansion in vitro of activated CD8 T cells was impaired. Novel homozygous or compound heterozygous deletions and point mutations in the gene encoding the dedicator of cytokinesis 8 protein (DOCK8) led to the absence of DOCK8 protein in lymphocytes.
Conclusions: Autosomal recessive DOCK8 deficiency is associated with a novel variant of combined immunodeficiency.
Massachusetts Medical Society
Conflict of interest statement
No potential conflict of interest relevant to this article was reported.
Figures

References
-
- Fischer A. Human primary immunodeficiency diseases. Immunity. 2007;27:835–45. - PubMed
-
- Bustamante J, Zhang SY, von Bernuth H, Abel L, Casanova JL. From infectious diseases to primary immunodeficiencies. Immunol Allergy Clin North Am. 2008;28:235–58. - PubMed
-
- Prawer SE, Pass F, Vance JC, Greenberg LJ, Yunis EJ, Zelickson AS. Depressed immune function in epidermodysplasia verruciformis. Arch Dermatol. 1977;113:495–9. - PubMed
-
- Renner ED, Puck JM, Holland SM, et al. Autosomal recessive hyperimmunoglobulin E syndrome: a distinct disease entity. J Pediatr. 2004;144:93–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
