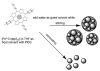
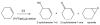Enhanced catalytic activity and unexpected products from the oxidation of cyclohexene by organic nanoparticles of 5,10,15,20-tetrakis-(2,3,4,5,6-pentafluorophenyl)porphyrinatoiron(III) in water by using O2
- PMID: 19777510
- PMCID: PMC2909443
- DOI: 10.1002/chem.200901086
Enhanced catalytic activity and unexpected products from the oxidation of cyclohexene by organic nanoparticles of 5,10,15,20-tetrakis-(2,3,4,5,6-pentafluorophenyl)porphyrinatoiron(III) in water by using O2
Abstract
The catalytic oxidation of alkenes by most iron porphyrins using a variety of oxygen sources, but generally not dioxygen, yields the epoxide with minor quantities of other products. The turnover numbers for these catalysts are modest, ranging from a few hundred to a few thousand depending on the porphyrin structure, axial ligands, and other reaction conditions. Halogenation of substituents increases the activity of the metalloporphyrin catalyst and/or makes it more robust to oxidative degradation. Oxidation of cyclohexene by 5,10,15,20-tetrakis-(2,3,4,5,6-pentafluorophenyl)porphyrinato iron(III), ([Fe(III)(tppf(20))]) and H(2)O(2) is typical of the latter: the epoxide is 99 % of the product and turnover numbers are about 350.1-4 Herein, we report that dynamic organic nanoparticles (ONPs) of [Fe(III)(tppf(20))] with a diameter of 10 nm, formed by host-guest solvent methods, catalytically oxidize cyclohexene with O(2) to yield only 2-cyclohexene-1-one and 2-cyclohexene-1-ol with approximately 10-fold greater turnover numbers compared to the non-aggregated metalloporphyrin in acetonitrile/methanol. These ONPs facilitate a greener reaction because the reaction solvent is 89 % water and O(2) is the oxidant in place of synthetic oxygen sources. This reactivity is unexpected because the metalloporphyrins are in close proximity and oxidative degradation of the catalyst should be enhanced, thus causing a significant decrease in catalytic turnovers. The allylic products suggest a different oxidative mechanism compared to that of the solvated metalloporphyrins. These results illustrate the unique properties of some ONPs relative to the component molecules or those attached to supports.
Figures
Similar articles
-
Adaptive organic nanoparticles of a teflon-coated iron (III) porphyrin catalytically activate dioxygen for cyclohexene oxidation.Macromol Rapid Commun. 2012 Jul 26;33(14):1220-6. doi: 10.1002/marc.201200107. Epub 2012 Apr 20. Macromol Rapid Commun. 2012. PMID: 22517679 Free PMC article.
-
Photoinitiated catalysis in Nafion membranes containing palladium(II) meso-tetrakis(N-methyl-4-pyridyl)porphyrin and iron(III) meso-tetrakis-(2,6-dichlorophenyl)porphyrin for O2-mediated oxidations of alkenes.Chemistry. 2001 Aug 17;7(16):3564-71. doi: 10.1002/1521-3765(20010817)7:16<3564::aid-chem3564>3.0.co;2-2. Chemistry. 2001. PMID: 11560328
-
Remarkable solvent, porphyrin ligand, and substrate effects on participation of multiple active oxidants in manganese(III) porphyrin catalyzed oxidation reactions.Chemistry. 2013 Jan 28;19(5):1810-8. doi: 10.1002/chem.201202640. Epub 2012 Nov 23. Chemistry. 2013. PMID: 23180447
-
First success of catalytic epoxidation of olefins by an electron-rich iron(III) porphyrin complex and H2O2: imidazole effect on the activation of H2O2 by iron porphyrin complexes in aprotic solvent.J Inorg Biochem. 2000 Jul 1;80(3-4):219-25. doi: 10.1016/s0162-0134(00)00085-4. J Inorg Biochem. 2000. PMID: 11001092
-
Differential coordination demands in Fe versus Mn water-soluble cationic metalloporphyrins translate into remarkably different aqueous redox chemistry and biology.Inorg Chem. 2013 May 20;52(10):5677-91. doi: 10.1021/ic3012519. Epub 2013 May 6. Inorg Chem. 2013. PMID: 23646875 Free PMC article.
Cited by
-
Selective Catalytic Oxidation of Cyclohexene with Molecular Oxygen: Radical Versus Nonradical Pathways.ChemCatChem. 2018 Mar 7;10(5):1035-1041. doi: 10.1002/cctc.201701538. Epub 2018 Jan 26. ChemCatChem. 2018. PMID: 29610628 Free PMC article.
-
Glycosylated Porphyrins, Phthalocyanines, and Other Porphyrinoids for Diagnostics and Therapeutics.Chem Rev. 2015 Sep 23;115(18):10261-306. doi: 10.1021/acs.chemrev.5b00244. Epub 2015 Aug 28. Chem Rev. 2015. PMID: 26317756 Free PMC article. Review.
-
Full use of factors promoting catalytic performance of chitosan supported manganese porphyrin.Sci Rep. 2020 Aug 24;10(1):14132. doi: 10.1038/s41598-020-70210-y. Sci Rep. 2020. PMID: 32839460 Free PMC article.
-
Adaptive organic nanoparticles of a teflon-coated iron (III) porphyrin catalytically activate dioxygen for cyclohexene oxidation.Macromol Rapid Commun. 2012 Jul 26;33(14):1220-6. doi: 10.1002/marc.201200107. Epub 2012 Apr 20. Macromol Rapid Commun. 2012. PMID: 22517679 Free PMC article.
-
Fluorinated porphyrinoids as efficient platforms for new photonic materials, sensors, and therapeutics.Org Biomol Chem. 2016 Jan 14;14(2):389-408. doi: 10.1039/c5ob01839k. Org Biomol Chem. 2016. PMID: 26514229 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources