The identity of the nucleophile substitution may influence metal interactions with the cleavage site of the minimal hammerhead ribozyme
- PMID: 19778032
- PMCID: PMC2901799
- DOI: 10.1021/bi900614v
The identity of the nucleophile substitution may influence metal interactions with the cleavage site of the minimal hammerhead ribozyme
Abstract
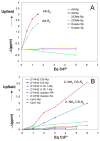
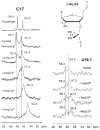
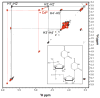
Potential metal interactions with the cleavage site of a minimal hammerhead ribozyme (mHHRz) were probed using (31)P NMR-detected Cd(2+) titration studies of HHRz constructs containing a phosphorothioate (PS) modification at the cleavage site. The mHHRz nucleophile position was replaced by either a 2'-F or a 2'-NH(2) in order to block cleavage activity during the study. The 2'-F/PS cleavage site mHHRz construct, in which the 2'-F should closely imitate the atom size and electronegativity of a 2'-OH, demonstrates low levels of metal ion association (<1 ppm (31)P chemical shift changes). This observation indicates that having an atom size and electrostatic properties that are similar to the 2'-OH are not the governing factors in allowing metal interactions with the scissile phosphate of the mHHRz. With a 2'-NH(2) substitution, a large upfield change in (31)P NMR chemical shift of the phosphorothioate peak (Delta approximately 3 ppm with 6 equiv of added Cd(2+)) indicates observable Cd(2+) interactions with the substituted site. Since a 2'-NH(2), but not a 2'-F, can serve as a metal ligand, these data suggest that a metal ion interaction with the HHRz cleavage site may include both the scissile phosphate and the 2' nucleophile. Control samples in which the 2'-NH(2)/PS unit is placed either next to the mHHRz cleavage site (at U16.1), in a duplex, or in a (am)U(PS)U dinucleotide show much weaker interactions with Cd(2+). Results with these control samples indicate that simply the presence of a 2'-NH(2)/PS unit does not create a strong metal binding site, reinforcing the possibility that the 2'-NH(2)-moderated Cd-PS interaction is specific to the mHHRz cleavage site. Upfield chemical shifts of both (31)P and H-2' (1)H resonances in (am)U(PS)U are observed with addition of Cd(2+), consistent with the predicted metal coordination to both 2'-NH(2) and phosphorothioate ligands. These data suggest that metal ion association with the HHRz cleavage site may include an interaction with the 2'-OH nucleophile.
Figures


Similar articles
-
Metal-phosphate interactions in the hammerhead ribozyme observed by 31P NMR and phosphorothioate substitutions.Biochemistry. 2000 Oct 10;39(40):12113-20. doi: 10.1021/bi001249w. Biochemistry. 2000. PMID: 11015188
-
A reappraisal, based on (31)P NMR, of the direct coordination of a metal ion with the phosphoryl oxygen at the cleavage site of a hammerhead ribozyme.J Am Chem Soc. 2002 Jul 17;124(28):8230-6. doi: 10.1021/ja0202098. J Am Chem Soc. 2002. PMID: 12105900
-
Identification of the hammerhead ribozyme metal ion binding site responsible for rescue of the deleterious effect of a cleavage site phosphorothioate.Biochemistry. 1999 Oct 26;38(43):14363-78. doi: 10.1021/bi9913202. Biochemistry. 1999. PMID: 10572011
-
Two distinct catalytic strategies in the hepatitis δ virus ribozyme cleavage reaction.Biochemistry. 2011 Nov 8;50(44):9424-33. doi: 10.1021/bi201157t. Epub 2011 Oct 17. Biochemistry. 2011. PMID: 22003985 Free PMC article. Review.
-
Folding and activity of the hammerhead ribozyme.Chembiochem. 2002 Aug 2;3(8):690-700. doi: 10.1002/1439-7633(20020802)3:8<690::AID-CBIC690>3.0.CO;2-C. Chembiochem. 2002. PMID: 12203967 Review.
Cited by
-
Divalent Metal Ion Activation of a Guanine General Base in the Hammerhead Ribozyme: Insights from Molecular Simulations.Biochemistry. 2017 Jun 20;56(24):2985-2994. doi: 10.1021/acs.biochem.6b01192. Epub 2017 Jun 12. Biochemistry. 2017. PMID: 28530384 Free PMC article.
-
Active participation of Mg ion in the reaction coordinate of RNA self-cleavage catalyzed by the hammerhead ribozyme.J Chem Theory Comput. 2011 Jan 11;7(1):1-3. doi: 10.1021/ct100467t. J Chem Theory Comput. 2011. PMID: 21379373 Free PMC article. No abstract available.
-
Hammerhead ribozymes: true metal or nucleobase catalysis? Where is the catalytic power from?Molecules. 2010 Aug 6;15(8):5389-407. doi: 10.3390/molecules15085389. Molecules. 2010. PMID: 20714304 Free PMC article.
-
Dynamic nuclear polarization of nucleic acid with endogenously bound manganese.J Biomol NMR. 2015 Sep;63(1):97-109. doi: 10.1007/s10858-015-9972-1. Epub 2015 Jul 29. J Biomol NMR. 2015. PMID: 26219517
-
A hammerhead ribozyme selects mechanically stable conformations for catalysis against viral RNA.Commun Biol. 2025 Feb 3;8(1):165. doi: 10.1038/s42003-025-07600-3. Commun Biol. 2025. PMID: 39900966 Free PMC article.
References
-
- Forster AC, Symons RH. Self-cleavage of virusoid RNA is performed by the proposed 55-nucleotide active site. Cell. 1987;50:9–16. - PubMed
-
- Haseloff J, Gerlach WL. Simple RNA enzymes with new and highly specific endoribonuclease activities. Nature. 1988;334:585–591. - PubMed
-
- Uhlenbeck OC. A small catalytic oligoribonucleotide. Nature. 1987;328:596–600. - PubMed
-
- Prody GA, Bakos JT, Buzayan JM, Schneider IR, Bruening G. Autolytic processing of dimeric plant-virus satellite RNA. Science. 1986;231:1577–1580. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

