Precise subcellular input retinotopy and its computational consequences in an identified visual interneuron
- PMID: 19778511
- PMCID: PMC2774212
- DOI: 10.1016/j.neuron.2009.09.010
Precise subcellular input retinotopy and its computational consequences in an identified visual interneuron
Abstract
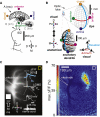
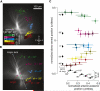
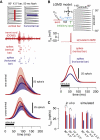
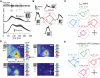
The Lobula Giant Movement Detector (LGMD) is a higher-order visual interneuron of Orthopteran insects that responds preferentially to objects approaching on a collision course. It receives excitatory input from an entire visual hemifield that anatomical evidence suggests is retinotopic. We show that this excitatory projection activates calcium-permeable nicotinic acetylcholine receptors. In vivo calcium imaging reveals that the excitatory projection preserves retinotopy down to the level of a single ommatidium. Examining the impact of retinotopy on the LGMD's computational properties, we show that sublinear synaptic summation can explain orientation preference in this cell. Exploring retinotopy's impact on directional selectivity leads us to infer that the excitatory input to the LGMD is intrinsically directionally selective. Our results show that precise retinotopy has implications for the dendritic integration of visual information in a single neuron.
Figures


Similar articles
-
Fine and distributed subcellular retinotopy of excitatory inputs to the dendritic tree of a collision-detecting neuron.J Neurophysiol. 2016 Jun 1;115(6):3101-12. doi: 10.1152/jn.00044.2016. Epub 2016 Mar 23. J Neurophysiol. 2016. PMID: 27009157 Free PMC article.
-
Time-dependent activation of feed-forward inhibition in a looming-sensitive neuron.J Neurophysiol. 2005 Sep;94(3):2150-61. doi: 10.1152/jn.00411.2005. Epub 2005 May 31. J Neurophysiol. 2005. PMID: 15928055 Free PMC article.
-
Synchronized neural input shapes stimulus selectivity in a collision-detecting neuron.Curr Biol. 2010 Nov 23;20(22):2052-7. doi: 10.1016/j.cub.2010.10.025. Epub 2010 Nov 4. Curr Biol. 2010. PMID: 21055939 Free PMC article.
-
Inhibitory circuits for visual processing in thalamus.Curr Opin Neurobiol. 2011 Oct;21(5):726-33. doi: 10.1016/j.conb.2011.06.004. Epub 2011 Jul 13. Curr Opin Neurobiol. 2011. PMID: 21752634 Free PMC article. Review.
-
Cellular mechanisms for direction selectivity in the retina.Neuron. 2007 Jul 19;55(2):179-86. doi: 10.1016/j.neuron.2007.07.001. Neuron. 2007. PMID: 17640521 Review.
Cited by
-
Logarithmic compression of sensory signals within the dendritic tree of a collision-sensitive neuron.J Neurosci. 2012 Apr 4;32(14):4923-34. doi: 10.1523/JNEUROSCI.5777-11.2012. J Neurosci. 2012. PMID: 22492048 Free PMC article.
-
Neural circuits underlying habituation of visually evoked escape behaviors in larval zebrafish.Elife. 2023 Mar 14;12:e82916. doi: 10.7554/eLife.82916. Elife. 2023. PMID: 36916795 Free PMC article.
-
Biophysics of object segmentation in a collision-detecting neuron.Elife. 2018 Apr 18;7:e34238. doi: 10.7554/eLife.34238. Elife. 2018. PMID: 29667927 Free PMC article.
-
Multiple clusters of release sites formed by individual thalamic afferents onto cortical interneurons ensure reliable transmission.Neuron. 2011 Jul 14;71(1):180-94. doi: 10.1016/j.neuron.2011.05.032. Neuron. 2011. PMID: 21745647 Free PMC article.
-
Tonotopic Ca2+ dynamics and sound processing in auditory interneurons of the bush-cricket Mecopoda elongata.J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2024 May;210(3):353-369. doi: 10.1007/s00359-023-01638-6. Epub 2023 May 24. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2024. PMID: 37222786 Free PMC article.
References
-
- Anderson JC, Binzegger T, Kahana O, Martin KA, Segev I. Dendritic asymmetry cannot account for directional responses of neurons in visual cortex. Nat Neurosci. 1999;2:820–4. - PubMed
-
- Baden T, Hedwig B. Neurite-specific Ca2+ dynamics underlying sound processing in an auditory interneurone. Dev Neurobiol. 2007;67:68–80. - PubMed
-
- Borst A, Egelhaaf M, Haag J. Mechanisms of dendritic integration underlying gain control in fly motion-sensitive interneurons. J Comput Neurosci. 1995;2:5–18. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

