Dynamics of fast and slow inhibition from cerebellar golgi cells allow flexible control of synaptic integration
- PMID: 19778512
- PMCID: PMC3595538
- DOI: 10.1016/j.neuron.2009.09.004
Dynamics of fast and slow inhibition from cerebellar golgi cells allow flexible control of synaptic integration
Abstract
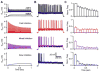
Throughout the brain, multiple interneuron types influence distinct aspects of synaptic processing. Interneuron diversity can thereby promote differential firing from neurons receiving common excitation. In contrast, Golgi cells are the sole interneurons regulating granule cell spiking evoked by mossy fibers, thereby gating inputs to the cerebellar cortex. Here, we examine how this single interneuron class modifies activity in its targets. We find that GABA(A)-mediated transmission at unitary Golgi cell --> granule cell synapses consists of varying contributions of fast synaptic currents and sustained inhibition. Fast IPSCs depress and slow IPSCs gradually build during high-frequency Golgi cell activity. Consequently, fast and slow inhibition differentially influence granule cell spike timing during persistent mossy fiber input. Furthermore, slow inhibition reduces the gain of the mossy fiber --> granule cell input-output curve, while fast inhibition increases the threshold. Thus, a lack of interneuron diversity need not prevent flexible inhibitory control of synaptic processing.
Figures
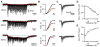



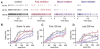
Comment in
-
Computational sophistication at a single GABAergic connection.Neuron. 2009 Sep 24;63(6):716-8. doi: 10.1016/j.neuron.2009.09.012. Neuron. 2009. PMID: 19778499
Similar articles
-
Synaptic and cellular properties of the feedforward inhibitory circuit within the input layer of the cerebellar cortex.J Neurosci. 2008 Sep 3;28(36):8955-67. doi: 10.1523/JNEUROSCI.5469-07.2008. J Neurosci. 2008. PMID: 18768689 Free PMC article.
-
Identification of an inhibitory circuit that regulates cerebellar Golgi cell activity.Neuron. 2012 Jan 12;73(1):149-58. doi: 10.1016/j.neuron.2011.10.030. Neuron. 2012. PMID: 22243753 Free PMC article.
-
Hyperpolarization induces a long-term increase in the spontaneous firing rate of cerebellar Golgi cells.J Neurosci. 2013 Apr 3;33(14):5895-902. doi: 10.1523/JNEUROSCI.4052-12.2013. J Neurosci. 2013. PMID: 23554471 Free PMC article.
-
Sensory representations in cerebellar granule cells.Curr Opin Neurobiol. 2009 Aug;19(4):445-51. doi: 10.1016/j.conb.2009.07.003. Epub 2009 Aug 3. Curr Opin Neurobiol. 2009. PMID: 19651506 Review.
-
Accounting for uncertainty: inhibition for neural inference in the cerebellum.Proc Biol Sci. 2021 Mar 31;288(1947):20210276. doi: 10.1098/rspb.2021.0276. Epub 2021 Mar 24. Proc Biol Sci. 2021. PMID: 33757352 Free PMC article. Review.
Cited by
-
Na+/K+-ATPase inhibition partially mimics the ethanol-induced increase of the Golgi cell-dependent component of the tonic GABAergic current in rat cerebellar granule cells.PLoS One. 2013;8(1):e55673. doi: 10.1371/journal.pone.0055673. Epub 2013 Jan 31. PLoS One. 2013. PMID: 23383260 Free PMC article.
-
Pathway-Specific Drive of Cerebellar Golgi Cells Reveals Integrative Rules of Cortical Inhibition.J Neurosci. 2019 Feb 13;39(7):1169-1181. doi: 10.1523/JNEUROSCI.1448-18.2018. Epub 2018 Dec 26. J Neurosci. 2019. PMID: 30587539 Free PMC article.
-
Repeated intermittent alcohol exposure during the third trimester-equivalent increases expression of the GABA(A) receptor δ subunit in cerebellar granule neurons and delays motor development in rats.Neuropharmacology. 2014 Apr;79:262-74. doi: 10.1016/j.neuropharm.2013.11.020. Epub 2013 Dec 4. Neuropharmacology. 2014. PMID: 24316160 Free PMC article.
-
Using c-kit to genetically target cerebellar molecular layer interneurons in adult mice.PLoS One. 2017 Jun 28;12(6):e0179347. doi: 10.1371/journal.pone.0179347. eCollection 2017. PLoS One. 2017. PMID: 28658323 Free PMC article.
-
Increased understanding of complex neuronal circuits in the cerebellar cortex.Front Cell Neurosci. 2024 Oct 21;18:1487362. doi: 10.3389/fncel.2024.1487362. eCollection 2024. Front Cell Neurosci. 2024. PMID: 39497921 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

