DGAT1 expression increases heart triglyceride content but ameliorates lipotoxicity
- PMID: 19778901
- PMCID: PMC2794747
- DOI: 10.1074/jbc.M109.049817
DGAT1 expression increases heart triglyceride content but ameliorates lipotoxicity
Abstract
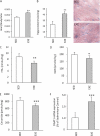
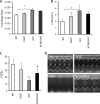
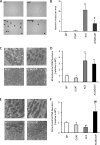
Intracellular lipid accumulation in the heart is associated with cardiomyopathy, yet the precise role of triglyceride (TG) remains unclear. With exercise, wild type hearts develop physiologic hypertrophy. This was associated with greater TG stores and a marked induction of the TG-synthesizing enzyme diacylglycerol (DAG) acyltransferase 1 (DGAT1). Transgenic overexpression of DGAT1 in the heart using the cardiomyocyte- specific alpha-myosin heavy chain (MHC) promoter led to approximately a doubling of DGAT activity and TG content and reductions of approximately 35% in cardiac ceramide, 26% in DAG, and 20% in free fatty acid levels. Cardiac function assessed by echocardiography and cardiac catheterization was unaffected. These mice were then crossed with animals expressing long-chain acyl-CoA synthetase via the MHC promoter (MHC-ACS), which develop lipotoxic cardiomyopathy. MHC-DGAT1XMHC-ACS double transgenic male mice had improved heart function; fractional shortening increased by 74%, and diastolic function improved compared with MHC-ACS mice. The improvement of heart function correlated with a reduction in cardiac DAG and ceramide and reduced cardiomyocyte apoptosis but increased fatty acid oxidation. In addition, the survival of the mice was improved. Our study indicates that TG is not likely to be a toxic lipid species directly, but rather it is a feature of physiologic hypertrophy and may serve a cytoprotective role in lipid overload states. Moreover, induction of DGAT1 could be beneficial in the setting of excess heart accumulation of toxic lipids.
Figures



References
-
- Yen C. L., Monetti M., Burri B. J., Farese R. V., Jr. (2005) J. Lipid Res. 46, 1502–1511 - PubMed
-
- Oelkers P., Behari A., Cromley D., Billheimer J. T., Sturley S. L. (1998) J. Biol. Chem. 273, 26765–26771 - PubMed
-
- Lardizabal K. D., Mai J. T., Wagner N. W., Wyrick A., Voelker T., Hawkins D. J. (2001) J. Biol. Chem. 276, 38862–38869 - PubMed
-
- Meegalla R. L., Billheimer J. T., Cheng D. (2002) Biochem. Biophys. Res. Commun. 298, 317–323 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

