PKA-dependent phosphorylation of serum response factor inhibits smooth muscle-specific gene expression
- PMID: 19778940
- PMCID: PMC2783385
- DOI: 10.1161/ATVBAHA.109.197285
PKA-dependent phosphorylation of serum response factor inhibits smooth muscle-specific gene expression
Abstract
Objective: Our goal was to identify phosphorylation sites that regulate serum response factor (SRF) activity to gain a better understanding of the signaling mechanisms that regulate SRF's involvement in smooth muscle cell (SMC)-specific and early response gene expression.
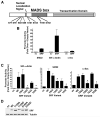
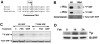
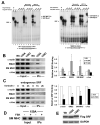
Methods and results: By screening phosphorylation-deficient and mimetic mutations in SRF(-/-) embryonic stem cells, we identified T159 as a phosphorylation site that significantly inhibits SMC-specific gene expression in an embryonic stem cell model of SMC differentiation. This residue conforms to a highly conserved consensus cAMP-dependent protein kinase (PKA) site, and in vitro and in vivo labeling studies demonstrated that it was phosphorylated by PKA. Results from gel shift and chromatin immunoprecipitation assays demonstrated that T159 phosphorylation inhibited SRF binding to SMC-specific CArG elements. Interestingly, the myocardin factors could at least partially rescue the effects of the T159D mutation under some conditions, but this response was promoter specific. Finally, PKA signaling had much less of an effect on c-fos promoter activity and SRF binding to the c-fos CArG.
Conclusions: Our results indicate that phosphorylation of SRF by PKA inhibits SMC-specific transcription suggesting a novel signaling mechanism for the control of SMC phenotype.
Figures



Similar articles
-
Coronary Disease-Associated Gene TCF21 Inhibits Smooth Muscle Cell Differentiation by Blocking the Myocardin-Serum Response Factor Pathway.Circ Res. 2020 Feb 14;126(4):517-529. doi: 10.1161/CIRCRESAHA.119.315968. Epub 2019 Dec 9. Circ Res. 2020. PMID: 31815603 Free PMC article.
-
Contribution of serum response factor and myocardin to transcriptional regulation of smoothelins.Cardiovasc Res. 2006 Apr 1;70(1):136-45. doi: 10.1016/j.cardiores.2005.12.018. Epub 2006 Jan 31. Cardiovasc Res. 2006. PMID: 16451796
-
Sphingosine 1-phosphate stimulates smooth muscle cell differentiation and proliferation by activating separate serum response factor co-factors.J Biol Chem. 2004 Oct 8;279(41):42422-30. doi: 10.1074/jbc.M405432200. Epub 2004 Aug 3. J Biol Chem. 2004. PMID: 15292266
-
Serum response factor: toggling between disparate programs of gene expression.J Mol Cell Cardiol. 2003 Jun;35(6):577-93. doi: 10.1016/s0022-2828(03)00110-x. J Mol Cell Cardiol. 2003. PMID: 12788374 Review.
-
Control of smooth muscle development by the myocardin family of transcriptional coactivators.Curr Opin Genet Dev. 2004 Oct;14(5):558-66. doi: 10.1016/j.gde.2004.08.003. Curr Opin Genet Dev. 2004. PMID: 15380248 Review.
Cited by
-
The STARS signaling pathway: a key regulator of skeletal muscle function.Pflugers Arch. 2014 Sep;466(9):1659-71. doi: 10.1007/s00424-014-1475-5. Epub 2014 Feb 21. Pflugers Arch. 2014. PMID: 24557714 Review.
-
Recent advances in serum response factor posttranslational modifications and their therapeutic potential in cardiovascular and neurological diseases.Vascul Pharmacol. 2024 Sep;156:107421. doi: 10.1016/j.vph.2024.107421. Epub 2024 Aug 30. Vascul Pharmacol. 2024. PMID: 39209126 Free PMC article. Review.
-
Noncanonical hedgehog pathway activation through SRF-MKL1 promotes drug resistance in basal cell carcinomas.Nat Med. 2018 Mar;24(3):271-281. doi: 10.1038/nm.4476. Epub 2018 Feb 5. Nat Med. 2018. PMID: 29400712 Free PMC article.
-
Olfactomedin 2 Regulates Smooth Muscle Phenotypic Modulation and Vascular Remodeling Through Mediating Runt-Related Transcription Factor 2 Binding to Serum Response Factor.Arterioscler Thromb Vasc Biol. 2017 Mar;37(3):446-454. doi: 10.1161/ATVBAHA.116.308606. Epub 2017 Jan 5. Arterioscler Thromb Vasc Biol. 2017. PMID: 28062493 Free PMC article.
-
cAMP regulation of airway smooth muscle function.Pulm Pharmacol Ther. 2013 Feb;26(1):112-20. doi: 10.1016/j.pupt.2012.05.007. Epub 2012 May 24. Pulm Pharmacol Ther. 2013. PMID: 22634112 Free PMC article. Review.
References
-
- Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 1993;362:801–9. - PubMed
-
- Owens GK, Kumar MS, Wamhoff BR. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol Rev. 2004;84:767–801. - PubMed
-
- Mack CP, Owens GK. Regulation of smooth muscle alpha-actin expression in vivo is dependent on CArG elements within the 5′ and first intron promoter regions. Circ Res. 1999;84:852–61. - PubMed
-
- Mack CP, Thompson MM, Lawrenz-Smith S, Owens GK. Smooth muscle alpha-actin CArG elements coordinate formation of a smooth muscle cell-selective, serum response factor-containing activation complex. Circ Res. 2000;86:221–32. - PubMed
-
- Madsen CS, Hershey JC, Hautmann MB, White SL, Owens GK. Expression of the smooth muscle myosin heavy chain gene is regulated by a negative-acting GC-rich element located between two positive-acting serum response factor-binding elements. J Biol Chem. 1997;272:6332–40. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

